DNA condensation
[1] DNA diameter is about 2 nm, while the length of a stretched single molecule may be up to several dozens of centimetres depending on the organism.
Many features of the DNA double helix contribute to its large stiffness, including the mechanical properties of the sugar-phosphate backbone, electrostatic repulsion between phosphates (DNA bears on average one elementary negative charge per each 0.17 nm of the double helix), stacking interactions between the bases of each individual strand, and strand-strand interactions.
Mathematically, for a non-interacting flexible chain randomly diffusing in 3D, the end-to-end distance would scale as a square root of the polymer length.
Although the double helices are always locally aligned, the DNA inside viruses does not represent real liquid crystals, because it lacks fluidity.
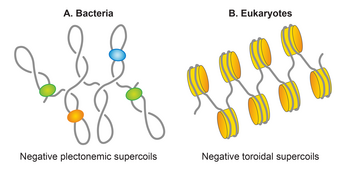
Protein-associated DNA occupies about 1/4 of the intracellular volume forming a concentrated viscous phase with liquid crystalline properties, called the nucleoid.
This convergence appears to depend on the ability of identical double-stranded DNA molecules to specifically identify each other, a process that culminates in the proximity of homologous sites along the paired chromosomes.
Diverse stress conditions appear to prime bacteria to effectively cope with severe DNA damages such as double-strand breaks.
The apposition of homologous sites associated with stress-induced chromosome condensation helps explain how repair of double-strand breaks and other damages occurs.
[6] Eukaryotic DNA with a typical length of dozens of centimeters should be orderly packed to be readily accessible inside the micrometer-size nucleus.
Chromosome scaffold is made of proteins including condensin, topoisomerase IIα and kinesin family member 4 (KIF4)[7] Dinoflagellates are very divergent eukaryotes in terms of how they package their DNA.
[ref] Since the double helices come very closely to each other in the condensed phase, this leads to the restructuring of water molecules, which gives rise to the so-called hydration forces.
[12] Nowadays descriptions of gene regulation are based on the approximations of equilibrium binding in dilute solutions, although it is clear that these assumptions are in fact violated in chromatin.