Danheiser benzannulation
The Danheiser benzannulation is a chemical reaction used in organic chemistry to generate highly substituted phenols in a single step.
A variety of substituted aromatic rings can be prepared using this method including: phenols, naphthalenes, benzofurans, benzothiophenes, indoles, and carbazoles.
[8] When ketenes are formed in the presence of alkynes they proceed through pericyclic reactions to generate a substituted aromatic ring (Scheme 2).
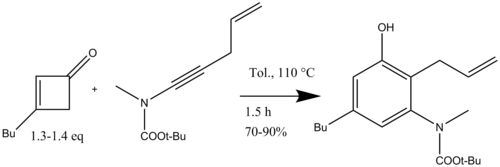
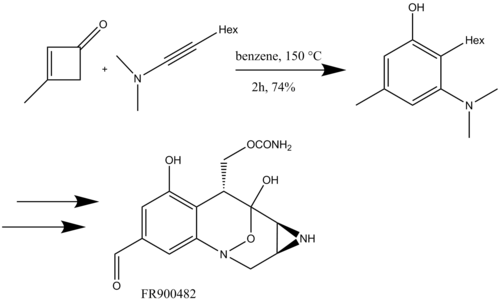
Avoiding the use of the high energy cyclobutenone starting materials provides access to a wider variety of substituted aromatic compounds.
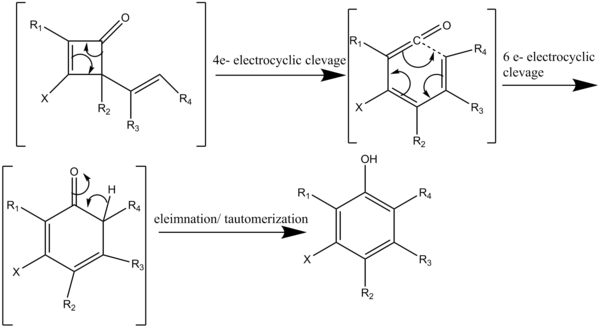
Heating a cyclobutenone above 80 °C initiates a four-electron electrocyclic cleavage generating a vinyl ketene which reacts with an acetylene in a regiospecific [2+2] cycloaddition (Scheme 4).
The dienylketene then undergoes a six-electron electrocyclization to give a hexadienone intermediate which rapidly tautomerizes to yield a highly substituted phenol or naphthol structures.
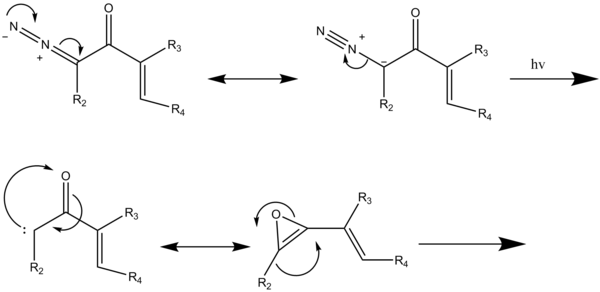
[5] In the case of the modified benzannulation reaction (Scheme 5); irradiation of the diazoketones induces the Wolff rearrangement yielding the vinyl ketene intermediate which reacts with the acetylene in a [2+2] cycloaddition then a four-electron cleavage of the resulting 4-substituted cyclobutenone produces a dienylketene which then undergoes a six-electron electrocyclization to give the 2,4-cyclohexanedione which tautomerizes to the final aromatic product.
A low-pressure mercury-vapor lamp at 254 nm in a photochemical reactor is used for 5–8 hours until all the diazoketone has been consumed as determined by TLC analysis.
[6] Diazoketones can be synthesized in one-step from readily available ketones or carboxylic acid precursors by the addition of diazomethane to acyl chlorides.
[2] The traditional method of the deformylative diazo transfer approach has been improved upon by substituting the trifluoroacetylation of generated lithium enolates for the Claisen formylation step.
The key step in this procedure is activation of the ketone starting material to the corresponding α-trifluoroacetyl derivative using trifluoroethyltrifluoroacetate (TFEA) (Scheme 9).
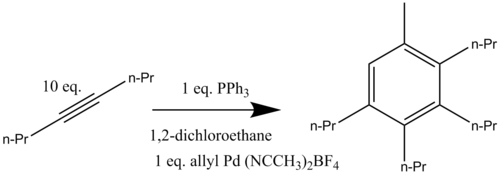
The cationic palladium complex [(η3-C3H5)Pd(CH3CN)2](BF4) reacts with an excess of 4-octyne when heated to 80 °C in the presence of triphenylphosphine forming the aromatic compound 1-methyl-2,3,4,5-tetrapropylbenzene (Scheme 12).
It was determined that the presence of exactly one equivalent of palladium catalyst (from which the allyl group adds into the final aromatic structure) is crucial for the catalyzed benzannulation to occur in good yield.
[17] Kowalski used the benzannulation reaction with siloxyacetylenes for the first time, reacting them with cyclobutenones to synthesize a substituted phenol for the total synthesis of Δ-6-tetrahydrocannabinol (Scheme 17).
[20] The successful usage of Danheiser benzannulation allows Zhang and Ready to achieve the so-far shortest synthesis of dictyodendrin natural products.