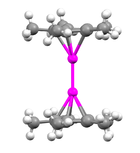
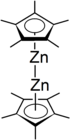Decamethyldizincocene
[2] Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water.
This compound appears to be indefinitely stable at room temperature (however storage at -20 °C is advised) and sublimes near 70 °C under vacuum.
[2] Various methods have been employed in order to determine the structure of decamethyldizincocene, including x-ray diffraction, 1H NMR, and mass spectrometry.
Through X-ray diffraction methods it has been found that the zinc atoms are sandwiched between two parallel C5Me5 rings whose planes are perpendicular to the metal-metal bond axis.
The C5Me5 rings are in an eclipsed conformation with the methyl substituents bent slightly outward (away from the central metal atoms) at angles of 3 to 6 degrees.
It is believed that in the case of decamethyldizincocene the bending of the methyl groups attached to the cyclopentadienyl ligands is preferential because it concentrates the electron density away from the central, positively charged metal atoms.
[7] The compound addressed in this paper is essentially linear with Zn-Zn bond angles of approximately 177°:[3] 1H NMR and mass spectrometry studies have been useful in proving that decamethyldizincocene does not include bridging ligands.
This study is important considering that the complex previously hypothesized to be Co2(η5-C5Me5)2 was later found using 1H NMR and mass spectrometry data to be supported by three bridging hydrogens.
[8] The 1H NMR of decamethyldizincocene shows only one signal at δ 2.02 due to the hydrogens attached to the methyl groups on the cyclopentadienyl ligands.