Dehydroepiandrosterone
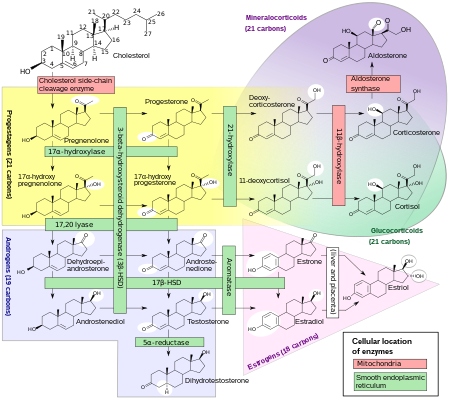
[7] It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues.
[12][13][14] DHEA is potentiated locally via conversion into testosterone and dihydrotestosterone (DHT) in the skin and hair follicles.
Remarkably however, DHEA acts as a full agonist of the ERβ with a maximal response similar to or actually slightly greater than that of estradiol, and its levels in circulation and local tissues in the human body are high enough to activate the receptor to the same degree as that seen with circulating estradiol levels at somewhat higher than their maximal, non-ovulatory concentrations; indeed, when combined with estradiol with both at levels equivalent to those of their physiological concentrations, overall activation of the ERβ was doubled.
[25][29][30] In any case, DHEA and DHEA-S both circulate at requisite concentrations to activate these receptors and were thus identified as important endogenous neurotrophic factors.
[31] Subsequent research has suggested that DHEA and/or DHEA-S may in fact be phylogenetically ancient "ancestral" ligands of the neurotrophin receptors from early on in the evolution of the nervous system.
[25][29] Similarly to pregnenolone, its synthetic derivative 3β-methoxypregnenolone (MAP-4343), and progesterone, DHEA has been found to bind to microtubule-associated protein 2 (MAP2), specifically the MAP2C subtype (Kd = 27 μM).
[33][34] It is thought that this action may possibly be responsible for much of the antiinflammatory, antihyperplastic, chemopreventative, antihyperlipidemic, antidiabetic, and antiobesic, as well as certain immunomodulating activities of DHEA (with some experimental evidence to support this notion available).
[42][52][53] This occurs naturally in the adrenal cortex and during first-pass metabolism in the liver and intestines when exogenous DHEA is administered orally.
[20] DHEA-S in turn can be converted back into DHEA in peripheral tissues via steroid sulfatase (STS).
[8] During pregnancy, DHEA-S is metabolized into the sulfates of 16α-hydroxy-DHEA and 15α-hydroxy-DHEA in the fetal liver as intermediates in the production of the estrogens estriol and estetrol, respectively.
[58] Prior to puberty in humans, DHEA and DHEA-S levels elevate upon differentiation of the zona reticularis of the adrenal cortex.
[25] Peak levels of DHEA and DHEA-S are observed around age 20, which is followed by an age-dependent decline throughout life eventually back to prepubertal concentrations.
[25] Levels of DHEA and DHEA-S decline to the lower nanomolar and micromolar ranges in men and women aged 60 to 80 years.
Women with polycystic ovary syndrome tend to have elevated levels of DHEA-S.[60] DHEA, also known as androst-5-en-3β-ol-17-one, is a naturally occurring androstane steroid and a 17-ketosteroid.
[61] The term "dehydroepiandrosterone" is ambiguous chemically because it does not include the specific positions within epiandrosterone at which hydrogen atoms are missing.