Dehydrogenase
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+[1] or a flavin coenzyme such as FAD or FMN.
[4] The name "dehydrogenase" is based on the idea that it facilitates the removal (de-) of hydrogen (-hydrogen-) and is an enzyme (-ase).
Note how when the hydride is transferred from A to B, the A has taken on a positive charge; this is because the enzyme has taken two electrons from the substrate in order to reduce the acceptor to BH.
[2] Another possibility is that a water molecule will enter the reaction, contributing a hydroxide ion to the substrate and a proton to the environment.
The distinction between the subclasses of oxidoreductases that catalyze oxidation reactions lies in their electron acceptors.
[6] Note that oxidases typically transfer the equivalent of dihydrogen (H2), and the acceptor is a dioxygen.
Similarly, a peroxidase (another subclass of oxidoreductases) will use a peroxide (H2O2) as the electron acceptor, rather than an oxygen.
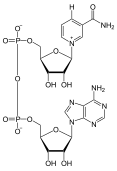
[5][7] Reduction of NAD+: NAD+ + 2H+ + 2e− ↔ NADH + H+NAD+ is mostly used in catabolic pathways, such as glycolysis, that break down energy molecules to produce ATP.
The ratio of NAD+ to NADH is kept very high in the cell, keeping it readily available to act as an oxidizing agent.
[8][9] These two electron carriers are easily distinguished by enzymes and participate in very different reactions.
The reasoning behind having two separate electron carriers for anabolic and catabolic pathways relates to regulation of metabolism.
Because it takes two atoms rather than one, FAD is often involved when a double bond is formed in the newly oxidized substrate.
Build-up of aldehydes in the brain and pericardium can be detrimental to a person's health, as they can form adducts with important molecules and cause their inactivation.
[16] These enzymes are only one example of the many different types of dehydrogenases in the human body; their wide array of functions, and the impact that their deactivation or mutations has upon crucial cell processes underscores the importance of all dehydrogenases in maintaining body homeostasis.