Swern oxidation
Of the volatile by-products, dimethyl sulfide has a strong, pervasive odour and carbon monoxide is acutely toxic, so the reaction and the work-up needs to be performed in a fume hood.
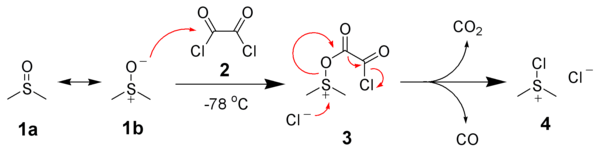
[8][9][10] The first step of the Swern oxidation is the low-temperature reaction of DMSO, 1a, formally as resonance contributor 1b, with oxalyl chloride, 2.
The addition of at least 2 equivalents of base — typically triethylamine — will deprotonate the alkoxysulfonium ion to give the sulfur ylide 7.
In a five-membered ring transition state, the sulfur ylide 7 decomposes to give dimethyl sulfide and the desired carbonyl compound 8.
For example, in Thompson & Heathcock's synthesis of the sesquiterpene isovelleral,[15] the final step uses the Swern protocol, avoiding rearrangement of the acid-sensitive cyclopropanemethanol moiety.