Radio-frequency welding
Advantages of this process are fast cycle times (on the order of a few seconds), automation, repeatability, and good weld appearance.
[1] When an alternating electrical field is applied, the molecule will continuously reverse its alignment, leading to molecular rotation.
As frequencies become high enough, power loss starts to increase as the dipoles are unable to align themselves at the rate of the reversing electrical field.
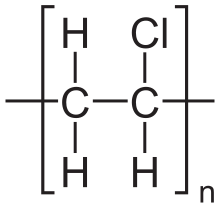
Like water, polyvinyl chloride (PVC) consists of asymmetrically distributed atoms of differing electronegativities, with a resulting dipole moment.
Because of its strong dipolar moment (and other properties) PVC is considered an excellent material for radio-frequency welding.
[1][2] Some plastics commonly welded with dielectric heating include:[1][3][6] Additional members can be added to a joint for a variety of reasons – improving thermal insulation, preventing sticking of parts to the welding equipment, preventing arcing, and buffering non-uniform clamping pressure or electric field.
Dielectric heating causes the parts that are in intimate contact to melt, and the liquid polymers diffuse into each other at the interface.
[2] Radio-frequency welding equipment, generally consists of: RF power generator, control unit, press, enclosure, electrodes, and sometimes a handling mechanism.
[1] An RF enclosure or a cage that goes around the electrodes and open areas is used to protect the operator from injury including radio frequency radiation.
[2] The most common application for RF welding is sealing thin sheets of polar thermoplastics such as PVC.
Some products that typically use RF welding include beach balls, airbeds, life jackets, book covers, and loose-leaf binders.
[3] RF welding is most typically used in the construction of products that require a watertight or airtight seal.