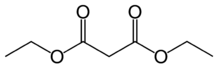
Diethyl malonate
It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes.
The extent of resonance stabilization of this compound's conjugate base is suggested by the three resonance forms below: Diethyl malonate is produced from the reaction of the sodium salt of chloroacetic acid with sodium cyanide, which produces the nitrile.
Only the "same" alkoxide anion as the one that one used to alkylate the deprotonated active methylenic site will prevent both base hydrolysis and transesterification.
DEAM can be acetylated to produce diethyl acetamidomalonate (useful in amino-acid synthesis), or can be added with 3-substituted 2,4-diketones to boiling acetic acid to afford in maximal yield variously substituted ethyl pyrrole-2-carboxylates of interest for porphyrin synthesis.
[7] Diethyl malonate is used in the preparation of several medicinally useful compounds including vigabatrin, phenylbutazone, nalidixic acid, and rebamipide.