Digital microfluidics
One example of this is the addition of electrochemiluminescence detectors within the indium tin oxide layer (the ground electrode in a closed system) which aid in the detection of luminophores in droplets.
[37] In their research, they attempted to model the Maxwell electrical charge acquired by a perfectly round conducting particle in the presence of a uniform magnetic field caused by a perfectly-conducting and infinitely-stretching surface.
[37] Their model helps to predict the Z-direction motion of the microdroplets within the device as it points to the magnitude and direction of forces acting upon a micro droplet.
A large benefit of employing this type of method is that it allows for two different environments to be accessible by the droplet, which can be taken advantage of by splitting the microfluidic tasks among the two surfaces.
[43][44] This device works very well with many liquids, including aqueous buffers, solutions of proteins and DNA, and undiluted bovine serum.
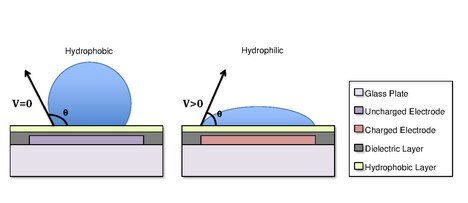
[46] In one of various embodiments of EWOD-based microfluidic biochips, investigated first by Cytonix in 1987 [1] Archived 2020-09-19 at the Wayback Machine and subsequently commercialized by Advanced Liquid Logic, there are two parallel glass plates.
A dielectric insulator coated with a hydrophobic is added to the plates to decrease the wet-ability of the surface and to add capacitance between the droplet and the control electrode.
In research fields such as synthetic biology, where highly iterative experimentation is common, considerable efforts have been made to automate workflows.
Workflows adapted from the bench to a DMF system are miniaturized, meaning working volumes are reduced to fractions of what is normally required for conventional methods.
For example, Thaitrong et al. developed a DMF system with a capillary electrophoresis (CE) module with the purpose of automating the process of next generation sequencing (NGS) library characterization.
Compared to an Agilent BioAnalyzer (an instrument commonly used to measure sequencing library size distribution), the DMF-CE system consumed ten-fold less sample volume.
Ruan et al. observed minimal contamination from exogenous nonhuman DNA and no cross-contamination between samples while using their DMF-based digital whole genome sequencing system.
[56] Using liquid handling robots that can minimize volume loss between experimental steps are often used to reduce error rates and improve reproducibility.
An automated DMF system for CRISPR-Cas9 genome editing was described by Sinha et al, and was used to culture and genetically modify H1299 lung cancer cells.
[50] This level of droplet control is important for workflows where reactions are sensitive to the order of reagent mixing and incubation times, but where the optimal values of these parameters may still need to be determined.
[77][80] Reduction, alkylation, and enzymatic digestion have also shown robustness and reproducibility utilizing DMF, indicating potential in the synthesis and manipulation of proteomics.
[73] Thus, conducting these syntheses on the microscale has the benefit of limiting money spent on purchasing reagents and waste products produced while yielding desirable experimental results.
There have been reports of reduced efficiency in chemical reactions as compared to bench-scale versions of the same syntheses, as lower product yields have been observed.
[80] Finally, samples are often subject to solvent evaporation which leads to changes in volume and concentration of reactants, and in some cases reactions to not go to completion.
Connecting the DMF chip to use in the field or world-to-chip interfaces have been accomplished by means of manual pumps and reservoirs which deliver microbes, cells, and media to the device.
[85] The lack of extensive pumps and valves allow for elaborate multi step applications involving cells performed in a simple and compact system.
[87] Another use of DMF platforms in cell culture is its ability to conduct in vitro cell-free cloning using single molecule PCR inside droplets.
[93][94][95][96] The incorporation of magnetic bead-based assays onto a DMF immunoassay platform has been demonstrated for the detection of multiple analytes, such as human insulin, IL-6, cardiac marker Troponin I (cTnI), thyroid stimulating hormone (TSH), sTNF-RI, and 17β-estradiol.
The ELISA template, commonly used for performing immunoassays and other enzyme-based biochemical assays, has been adapted for use with the DMF platform for the detection of analytes such as IgE and IgG.
[100][101] In one example,[93] a series of bioassays were conducted to establish the quantification capabilities of DMF devices, including an ELISA-based immunoassay for the detection of IgE.
[96][102][103] For example, Rackus et al.[102] integrated microelectrodes onto a DMF chip surface and substituted a previously reported chemiluminescent IgG immunoassay[104] with an electroactive species, enabling detection of rubella virus.
[105] Indirect off-line analysis is the usage of DMF devices to combine reactants and isolate products, which are then removed and manually transferred to a mass spectrometer.
[109][17] Some couplings utilize an external high-voltage pulse source at the physical inlet to the mass spectrometer [110] but the true role of such additions is uncertain.
MMS are optimized towards affordability and simple operation, often forgoing the need for experienced technicians, having a low cost of manufacture, and being small enough in size to allow for the transfer of data collection from the laboratory into the field.
In one example the use of a custom DMF system for urine drug testing enabled the creation of an instrument weighing only 25 kg with performance comparable to standard laboratory analysis.