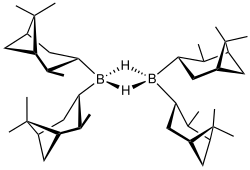
Diisopinocampheylborane
The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes.
[1] Diisopinocampheylborane was originally prepared by hydroboration of excess α-pinene with borane,[2] but it is now more commonly generated from borane-methyl sulfide (BMS).
[3] The compound can be isolated as a solid, but because it is quite sensitive to water and air, it is often generated in situ and used as a solution.
[1] Diisopinocampheylborane is often represented as a monomer (including in this article), but X-ray crystallography establishes a dimeric structure.
Methanolysis gives methoxydiisopinocampheylborane Because of the large size of the α-pinenyl substituents, diisopinocampheylborane only hydroborates unhindered alkenes.
[5] It adds to alkynes to form the corresponding vinyldiisopinocampheylboranes In a highly stereoselective reaction, allyldiisopinocampheylboranes converts aldehydes to the homologated alcohols, rapidly even at -100 °C.