Dimerization
In chemistry, dimerization is the process of joining two identical or similar molecular entities by bonds.
For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds.
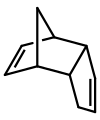
Dicyclopentadiene is an asymmetrical dimer of two cyclopentadiene molecules that have reacted in a Diels-Alder reaction to give the product.
Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers: Many nonmetallic elements occur as dimers: hydrogen, nitrogen, oxygen, and the halogens fluorine, chlorine, bromine and iodine.
Some metals form a proportion of dimers in their vapour phase: dilithium (Li2), disodium (Na2), dipotassium (K2), dirubidium (Rb2) and dicaesium (Cs2).
In the context of polymers, "dimer" also refers to the degree of polymerization 2, regardless of the stoichiometry or condensation reactions.
[7] Trialkylaluminium compounds can exist as either monomers or dimers, depending on the steric bulk of the groups attached.
[8] Cyclopentadienylchromium tricarbonyl dimer exists in measureable equilibrium quantities with the monometallic radical (C5H5)Cr(CO)3.
[8] When pyrimidine dimers are present, they can block polymerases, decreasing DNA functionality until it is repaired.