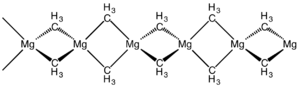
Dimethylmagnesium
Like other dialkylmagnesium compounds, dimethylmagnesium is prepared by adding dioxane to a solution of methylmagnesium halide:[3] In such procedures, the dimethylmagnesium exists as the ether adduct, not the polymer.
[4] Addition of 1,4-dioxane causes precipitation of solid MgX2(μ-dioxane)2, a coordination polymer.
Related methods have been applied to other dialkylmagnesium compounds.
The material is a polymer with the same connectivity as silicon disulfide, featuring tetrahedral magnesium centres, each surrounded by bridging methyl groups.
[7] The linear chain structure seen for dimethylmagnesium is also observed for diethylmagnesium and dimethylberyllium.