Group 2 organometallic chemistry
[2][3] By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry.
However, as the group two elements (with the exception of beryllium) have considerably low electronegativity the resulting C-M bonds are more highly polarized and ionic-like, if not entirely ionic for the heavier barium compounds.
The lighter organoberyllium and organomagnesium compounds are often considered covalent, but with some ionic bond characteristics owing to the attached carbon bearing a negative dipole moment.
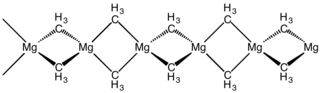
This higher ionic character and bond polarization tends to produce high coordination numbers and many compounds (particularly dialklys) are polymeric in solid or liquid states with highly complex structures in solution, though in the gaseous state they are often monomeric.
Bis(cyclopentadienyl)beryllium or beryllocene (Cp2Be), with a molecular dipole moment of 2.2 D, is so-called slipped 5η/1η sandwich.
While magnesocene (Cp2Mg) is a regular metallocene, bis(pentamethylcyclopentadienyl)calcium (Cp*)2Ca is bent with an angle of 147°.
[17] The distinctive feature of the Grignard reagents is their formation from the organic halide and magnesium metal.
Most other group II organic compounds are generated by salt metathesis, which limits their accessibility.
These simplified formulas are deceptive: Grignard reagents generally exist as dietherates, RMgX(ether)2.
[20] It forms in a metathesis reaction of allylpotassium and calcium iodide as a stable non-pyrophoric off-white powder: The bonding mode is η3.