Allotropes of oxygen
In outer space, the presence of ample ultraviolet radiation results in a low Earth orbit atmosphere in which 96% of the oxygen occurs in atomic form.
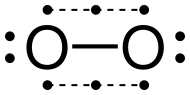
As a major component (about 21% by volume) of Earth's atmosphere, elemental oxygen is most commonly encountered in the diatomic form.
Aerobic organisms use atmospheric dioxygen as the terminal oxidant in cellular respiration in order to obtain chemical energy.
It was named "ozon" in 1840 by Christian Friedrich Schönbein,[8] from ancient Greek ὄζειν (ozein: "to smell") plus the suffix -on, commonly used at the time to designate a derived compound and anglicized as -one.
It is formed by reaction of intact O2 with atomic oxygen produced when UV radiation in the upper atmosphere splits O2.
[10] Ground-level ozone is an air pollutant that is especially harmful for senior citizens, children, and people with heart and lung conditions such as emphysema, bronchitis, and asthma.
[12] Cyclic ozone is a theoretically predicted O3 molecule in which its three atoms of oxygen bond in an equilateral triangle instead of an open angle.
Cacace's team suggested that O4 probably consists of two dumbbell-like O2 molecules loosely held together by induced dipole dispersion forces.