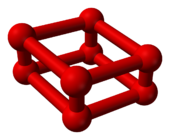
Octaoxygen
[1] As the pressure of oxygen at room temperature is increased above 10 gigapascals (1,500,000 psi), it undergoes a dramatic phase transition to a different allotrope.
Based on infrared spectroscopy, researchers assumed in 1999 that this phase consists of O4 molecules in a crystal lattice.
In this phase, it exhibits a dark-red color, very strong infrared absorption, and a magnetic collapse.
[8] It is also stable over a very large pressure domain[citation needed] and has been the subject of numerous X-ray diffraction, spectroscopic and theoretical studies.
It has been shown to have a monoclinic C2/m symmetry, and its infrared absorption behaviour was attributed to the association of oxygen molecules into larger units.