Triplet oxygen
Molecular orbital theory must be used to correctly account for the observed paramagnetism and short bond length simultaneously.
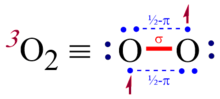
The usual rules for constructing Lewis structures must be modified to accommodate molecules like triplet dioxygen or nitric oxide that contain 2c-3e bonds.
There is no consensus in this regard; Pauling has suggested the use of three closely spaced collinear dots to represent the three-electron bond (see illustration).
The unusual electron configuration prevents molecular oxygen from reacting directly with many other molecules, which are often in the singlet state.
The extra energy required is sufficient to prevent direct reaction at ambient temperatures with all but the most reactive substrates, e.g. white phosphorus.
For instance, most flammable substances are characterised by an autoignition temperature at which they will undergo combustion in air without an external flame or spark.