Dioxygenyl
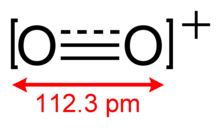
The dioxygenyl ion, O+2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2.
Neil Bartlett demonstrated that dioxygenyl hexafluoroplatinate (O2PtF6), containing the dioxygenyl cation, can be prepared at room temperature by direct reaction of oxygen gas (O2) with platinum hexafluoride (PtF6):[6] The compound can also be prepared from a mixture of fluorine and oxygen gases in the presence of a platinum sponge at 450 °C, and from oxygen difluoride (OF2) above 400 °C:[6] At lower temperatures (around 350 °C), platinum tetrafluoride is produced instead of dioxygenyl hexafluoroplatinate.
[7] O+2 is also found in similar compounds of the form O2MF6, where M is arsenic (As), antimony (Sb),[8] gold (Au),[9] niobium (Nb), ruthenium (Ru), rhenium (Re), rhodium (Rh),[10] vanadium (V),[11] or phosphorus (P).
[11] The tetrafluoroborate and hexafluorophosphate salts may be prepared by the reaction of dioxygen difluoride with boron trifluoride or phosphorus pentafluoride at −126 °C:[12] These compounds rapidly decompose at room temperature: Some compounds including O2Sn2F9, O2Sn2F9·0.9HF, O2GeF5·HF, and O2[Hg(HF)]4(SbF6)9 can be made by ultraviolet irradiation of oxygen and fluorine dissolved in anhydrous hydrogen fluoride with a metal oxide.
The reaction of O2BF4 with xenon at 173 K (−100 °C) produces a white solid believed to be F–Xe–BF2, containing an unusual xenon-boron bond:[14] The dioxygenyl salts O2BF4 and O2AsF6 react with carbon monoxide to give oxalyl fluoride, C2O2F2, in high yield.