Ionization energy
[3] Comparison of ionization energies of atoms in the periodic table reveals two periodic trends which follow the rules of Coulombic attraction:[4] The latter trend results from the outer electron shell being progressively farther from the nucleus, with the addition of one inner shell per row as one moves down the column.
[citation needed] Also, many solid elements can be heated and vaporized into single atoms.
Monatomic vapor is contained in a previously evacuated tube that has two parallel electrodes connected to a voltage source.
Some values for elements of the third period are given in the following table: Large jumps in the successive molar ionization energies occur when passing noble gas configurations.
Moving left to right within a period, or upward within a group, the first ionization energy generally increases,[9] with exceptions such as aluminium and sulfur in the table above.
On moving downward within a given group, the electrons are held in higher-energy shells with higher principal quantum number n, further from the nucleus and therefore are more loosely bound so that the ionization energy decreases.
This occurs because the outer electron in the alkali metals requires a much lower amount of energy to be removed from the atom than the inner shells.
[14][15][16] The trends and exceptions are summarized in the following subsections: Ionization energy values tend to decrease on going to heavier elements within a group[12] as shielding is provided by more electrons and overall, the valence shells experience a weaker attraction from the nucleus, attributed to the larger covalent radius which increase on going down a group[27] Nonetheless, this is not always the case.
The cloud's underlying mathematical representation is the wavefunction, which is built from Slater determinants consisting of molecular spin orbitals.
[41] These are related by Pauli's exclusion principle to the antisymmetrized products of the atomic or molecular orbitals.
Calculating these energies exactly is not possible except for the simplest systems (i.e. hydrogen and hydrogen-like elements), primarily because of difficulties in integrating the electron correlation terms.
Due to the possible changes in molecular geometry that may result from ionization, additional transitions may exist between the vibrational ground state of the neutral species and vibrational excited states of the positive ion.
The intensity of such transitions is explained by the Franck–Condon principle, which predicts that the most probable and intense transition corresponds to the vibrationally excited state of the positive ion that has the same geometry as the neutral molecule.
For a diatomic molecule, the geometry is defined by the length of a single bond.
In Figure 1, the lower potential energy curve is for the neutral molecule and the upper surface is for the positive ion.
This effect is represented by shifting the minimum of the potential energy curve to the right of the neutral species.
The adiabatic ionization is the diagonal transition to the vibrational ground state of the ion.
Vertical ionization may involve vibrational excitation of the ionic state and therefore requires greater energy.
While the term ionization energy is largely used only for gas-phase atomic, cationic, or molecular species, there are a number of analogous quantities that consider the amount of energy required to remove an electron from other physical systems.
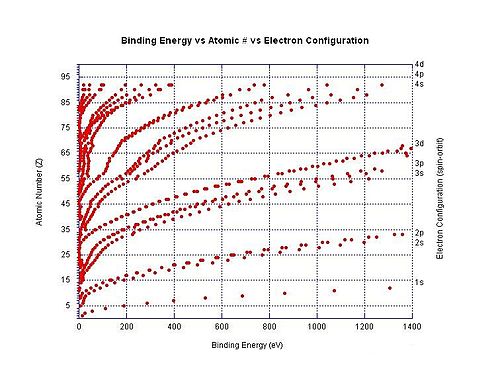
The graph to the right shows the binding energy for electrons in different shells in neutral atoms.
Work function is the minimum amount of energy required to remove an electron from a solid surface, where the work function W for a given surface is defined by the difference[47] where −e is the charge of an electron, ϕ is the electrostatic potential in the vacuum nearby the surface, and EF is the Fermi level (electrochemical potential of electrons) inside the material.