Dirigent protein
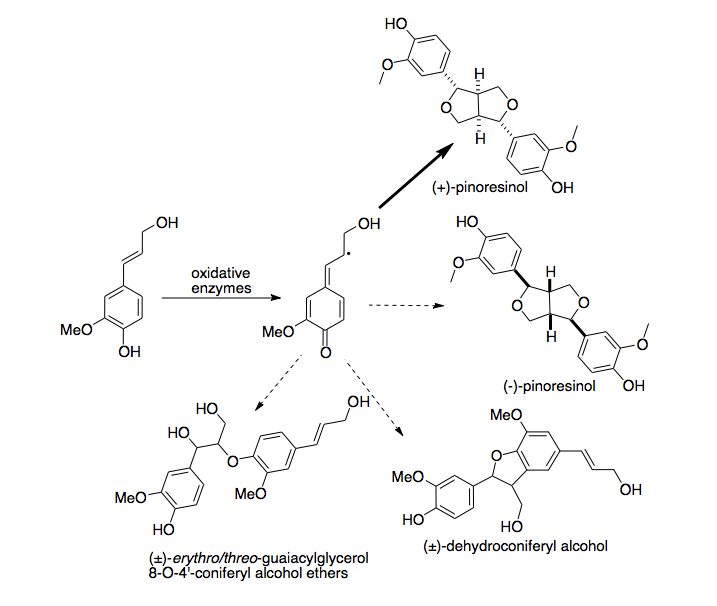
[5] Recently, a second, enantiocomplementary dirigent protein was identified in Arabidopsis thaliana, which directs enantioselective synthesis of (-)-pinoresinol.
[6] In lignan biosynthesis, oxidative enzymes perform proton coupled electron transfer to remove a hydrogen atom from monolignols, forming a radical intermediate.
[7] In vitro reactions of coniferyl alcohol (a common monolignol) in the presence of oxidative enzymes produce a wide variety of different dimers at varying concentrations.
[10] When other monolignols, such as p-coumaryl alcohol and sinapyl alcohol, are reacted in vitro with oxidative enzymes in the presence of dirigent protein, they produce a heterologous mixture of products indistinguishable from identical experiments in the absence of dirigent protein.
The biological significance of (+)-pinoresinol in plants is not fully understood, but it has been found to be effective as a feeding deterrent against ants in caterpillars of the cabbage butterfly, which obtain the compound from their diet.