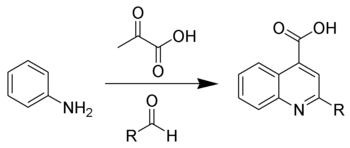
Doebner reaction
One possibility is at first an aldol condensation, starting from the enol form of the pyruvic acid (1) and the aldehyde, forming an β,γ-unsaturated α-ketocarboxylic acid (2).
After a cyclization at the benzene ring and two proton shifts, the quinoline-4-carboxylic acid (4) is formed by water elimination:[4]
The subsequent reaction with the enol form of pyruvic acid (1) leads to the formation of the above-mentioned aniline derivative (3) followed by the above-described reaction mechanism:[4]
It is reported in the literature that the Doebner reaction fails in case of 2-chloro-5-aminopyridine.
In this case the cyclization would take place at the amino group instead of the benzene ring and lead to a pyrrolidine derivative.