Doebner–Miller reaction
[1][2][3][4][5] This reaction is also known as the Skraup-Doebner-Von Miller quinoline synthesis, and is named after the Czech chemist Zdenko Hans Skraup (1850–1910), and the Germans Oscar Döbner (Doebner) (1850–1907) and Wilhelm von Miller (1848–1899).
A 2006 study [6] proposes a fragmentation-recombination mechanism based on carbon isotope scrambling experiments.
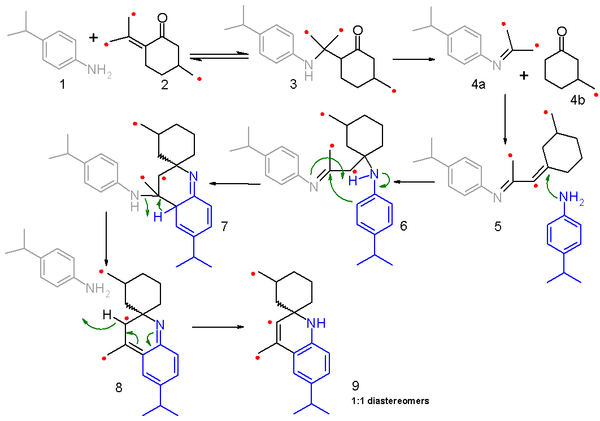
In this study 4-isopropylaniline 1 is reacted with a mixture (50:50)of ordinary pulegone and the 13C-enriched isomer 2 and the reaction mechanism is outlined in scheme 2 with the labeled carbon identified with a red dot.
In the next step 5 reacts with a second aniline molecule in a nucleophilic conjugate addition to imine 6 and subsequent electrophilic addition and proton transfer to leads to 7. elimination of one aniline molecule through 8 and rearomatization leads to final product 9.
Because α-amino protons are not available in this model compound the reaction is not taken to the fully fledged quinoline.