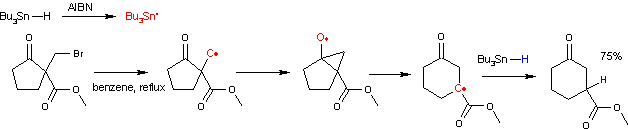
Dowd–Beckwith ring-expansion reaction
[1][2][3] The radical initiator system is based on azobisisobutyronitrile and tributyltin hydride.
This radical attacks the carbonyl group to an intermediate bicyclic ketyl.
A side reaction accompanying this ring expansion is organic reduction of the halo alkane to a saturated alkyl group.
One study [4] shows that the success depends critically on the accessibility of the carbonyl group.
The reaction of the alkyl radical with the ester carbonyl group is also a possibility but has an unfavorable activation energy.