E1cB-elimination reaction
The lone pair of electrons on the anion then moves to the neighboring atom, thus expelling the leaving group and forming a double or triple bond.
The E1 mechanism usually has the opposite characteristics: the leaving group is a good one (like -OTs or -Br), while the hydrogen is not particularly acidic and a strong base is absent.
Due to the presence of an empty p orbital after departure of the leaving group, the hydrogen on the neighboring carbon becomes much more acidic, allowing it to then be removed by the weak base in the second step.
In an E2 reaction, the presence of a strong base and a good leaving group allows proton abstraction by the base and the departure of the leaving group to occur simultaneously, leading to a concerted transition state in a one-step process.
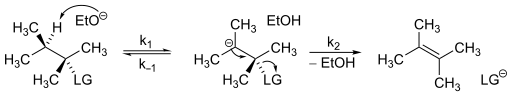
The compound must have an acidic hydrogen on its β-carbon and a relatively poor leaving group on the α- carbon.
The first step of an E1cB mechanism is the deprotonation of the β-carbon, resulting in the formation of an anionic transition state, such as a carbanion.
This transition state can be stabilized through induction or delocalization of the electron lone pair through resonance.
An example of an E1cB mechanism that has a stable transition state can be seen in the degradation of ethiofencarb - a carbamate insecticide that has a relatively short half-life in Earth's atmosphere.
Some examples of compounds that contain poor leaving groups and can undergo the E1cB mechanism are alcohols and fluoroalkanes.
Although the mechanisms are similar, they vary in the timing of the deprotonation of the α-carbon and the loss of the leaving group.
The molecule involved must also have a very good leaving group such as bromine or chlorine, and it should have a relatively less acidic α-carbon.
In an E2-elimination reaction, both the deprotonation of the α-carbon and the loss of the leaving group occur simultaneously in one concerted step.
If the solvent is protic and contains deuterium in place of hydrogen (e.g., CH3OD), then the exchange of protons into the starting material can be monitored.
Recall, in this mechanism protonation of the carbanion (either by the conjugate acid or by solvent) is faster than loss of the leaving group.
If the reactant contains deuterium at the β position, a primary kinetic isotope effect indicates that deprotonation is rate determining.
Fluorine kinetic isotope effects are also applied in the labeling of Radiopharmaceuticals and other compounds in medical research.