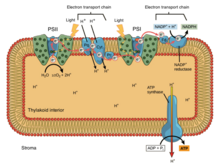
Electron transport chain
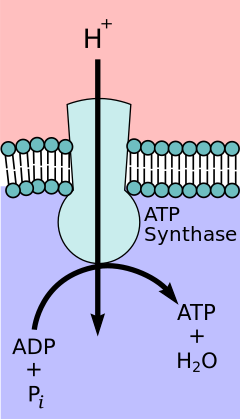
The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP).
In an electron transport chain, the redox reactions are driven by the difference in the Gibbs free energy of reactants and products.
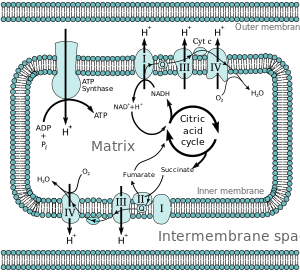
[2] In eukaryotic organisms, the electron transport chain, and site of oxidative phosphorylation, is found on the inner mitochondrial membrane.
The energy released by reactions of oxygen and reduced compounds such as cytochrome c and (indirectly) NADH and FADH2 is used by the electron transport chain to pump protons into the intermembrane space, generating the electrochemical gradient over the inner mitochondrial membrane.
Each reaction releases energy because a higher-energy donor and acceptor convert to lower-energy products.
[citation needed] Energy associated with the transfer of electrons down the electron transport chain is used to pump protons from the mitochondrial matrix into the intermembrane space, creating an electrochemical proton gradient (ΔpH) across the inner mitochondrial membrane.
This proton gradient is largely but not exclusively responsible for the mitochondrial membrane potential (ΔΨM).
[6] The pathway of electrons is as follows: NADH is oxidized to NAD+, by reducing flavin mononucleotide to FMNH2 in one two-electron step.
This current powers the active transport of four protons to the intermembrane space per two electrons from NADH.
Therefore, the pathway through Complex II contributes less energy to the overall electron transport chain process.
The two other electrons sequentially pass across the protein to the Qi site where the quinone part of ubiquinone is reduced to quinol.
[10] The FO component of ATP synthase acts as an ion channel that provides for a proton flux back into the mitochondrial matrix.
Protons in the inter-membrane space of mitochondria first enter the ATP synthase complex through an a subunit channel.
[11] The number of c subunits determines how many protons are required to make the FO turn one full revolution.
Usually requiring a significant amount of energy to be used, this can reduce the oxidized forms of electron donors.
One example is blockage of ATP synthase, resulting in a build-up of protons and therefore a higher proton-motive force, inducing reverse electron flow.
In other words, they correspond to successively smaller Gibbs free energy changes for the overall redox reaction.
A common feature of all electron transport chains is the presence of a proton pump to create an electrochemical gradient over a membrane.
Chemoorganotrophs (animals, fungi, protists) and photolithotrophs (plants and algae) constitute the vast majority of all familiar life forms.
Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, manganese oxide, and ferrous iron.
Lithotrophs have been found growing in rock formations thousands of meters below the surface of Earth.
The use of inorganic electron donors such as hydrogen as an energy source is of particular interest in the study of evolution.
This type of metabolism must logically have preceded the use of organic molecules and oxygen as an energy source.
[citation needed] Quinones are mobile, lipid-soluble carriers that shuttle electrons (and protons) between large, relatively immobile macromolecular complexes embedded in the membrane.
Mitochondrial Complex III is this second type of proton pump, which is mediated by a quinone (the Q cycle).
Some cytochromes are water-soluble carriers that shuttle electrons to and from large, immobile macromolecular structures imbedded in the membrane.
If oxygen is available, it is most often used as the terminal electron acceptor in aerobic bacteria and facultative anaerobes.
Under aerobic conditions, it uses two different terminal quinol oxidases (both proton pumps) to reduce oxygen to water.
[2] Mostly in anaerobic environments different electron acceptors are used, including nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules such as fumarate.
When bacteria grow in anaerobic environments, the terminal electron acceptor is reduced by an enzyme called a reductase.