Isocitrate dehydrogenase
In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria.
In comparing C. glutamicum and E. coli,[4] monomer and dimer, respectively, both enzymes were found to "efficiently catalyze identical reactions."
The dimer E. coli showed stability at a higher temperature than normal due to the interactions between the two monomeric subunits.
It is a homodimer in which each subunit has a Rossmann fold, and a common top domain of interlocking β sheets.
Mtb ICDH-1 is most structurally similar to the R132H mutant human ICDH found in CNS WHO grade 4 astrocytomas, formerly classified[5] as glioblastomas.
[6] The IDH step of the citric acid cycle is often (but not always) an irreversible reaction due to its large negative change in free energy.
[12] Within the citric acid cycle, isocitrate, produced from the isomerization of citrate, undergoes both oxidation and decarboxylation.
The oxidation of the alpha carbon introduces a molecular arrangement where electrons (in the next step) will flow from the nearby carboxyl group and push the electrons of the double bonded oxygen up onto the oxygen atom itself, which collects a proton from a nearby lysine.
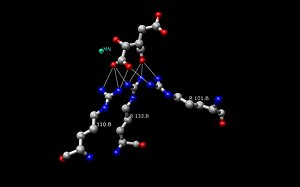
Two aspartate amino acid residues (below left) are interacting with two adjacent water molecules (w6 and w8) in the Mn2+ isocitrate porcine IDH complex to deprotonate the alcohol off the alpha-carbon atom.
In this reaction, the lone pair on the adjacent Tyrosine hydroxyl abstracts the proton off the carboxyl group.
The deprotonation of the carboxyl group causes the lone pair of electrons to move down making carbon dioxide and separating from oxalosuccinate.
The metal-ion forms a little complex through ionic interactions with the oxygen atoms on the fourth and fifth carbons (also known as the gamma subunit of isocitrate).
This lone pair of electrons abstracts a proton off the Tyrosine that deprotonated the carboxyl group in the decarboxylation step.
The reason that we can say that the Lys and Tyr residues will be the same from the previous step is because they are helping in holding the isocitrate molecule in the active site of the enzyme.
[14] Since then, the Escherichia coli IDH structure has been used by most researchers to make comparisons to other isocitrate dehydrogenase enzymes.
There is much detailed knowledge about this bacterial enzyme, and it has been found that most isocitrate dehydrogenases are similar in structure and therefore also in function.
Notarangelo et al. showed that such high concentrations of D-2HG could act as a direct inhibitor of lactate dehydrogenase in mouse T cells.