Aconitase
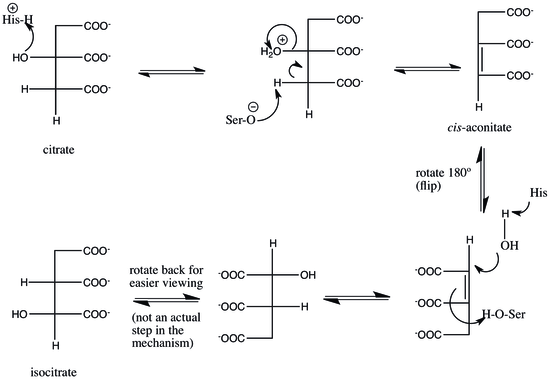
Aconitase (aconitate hydratase; EC 4.2.1.3) is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.
The iron-responsive element-binding protein (IRE-BP) and 3-isopropylmalate dehydratase (α-isopropylmalate isomerase; EC 4.2.1.33), an enzyme catalysing the second step in the biosynthesis of leucine, are known aconitase homologues.
The specific regulator protein, the IRE-BP, binds to IREs in both 5' and 3' regions, but only to RNA in the apo form, without the Fe-S cluster.
Mutant IRE-BPs, in which any or all of the three Cys residues involved in Fe-S formation are replaced by serine, have no aconitase activity, but retain RNA-binding properties.
One theory is that, in the rate-limiting step of the mechanism, the cis-aconitate is released from the enzyme, then reattached in the isocitrate mode to complete the reaction.
[11][12] To complete the reaction, the serine and histidine residues reverse their original catalytic actions: the histidine, now basic, abstracts a proton from water, priming it as a nucleophile to attack at C2, and the protonated serine is deprotonated by the cis-aconitate double bond to complete the hydration, producing isocitrate.
In citrus fruits, a reduction of the activity of the mitochondrial aconitases likely leads to the buildup of citric acid, which is then stored in vacuoles.
[18] Humans express the following two aconitase isozymes: Click on genes, proteins and metabolites below to link to respective articles.