Electroplating
Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion and corrosion, lubricity, reflectivity, electrical conductivity, or appearance.
It is used to build up thickness on undersized or worn-out parts and to manufacture metal plates with complex shape, a process called electroforming.
These free cyanides facilitate anode corrosion, help to maintain a constant metal ion level, and contribute to conductivity.
One example of this situation is the poor adhesion of electrolytic nickel on zinc alloys, in which case a copper strike is used, which has good adherence to both.
[7] The experimental parameters of pulse electroplating usually consist of peak current/potential, duty cycle, frequency, and effective current/potential.
Duty cycle is the effective portion of time in a certain electroplating period with the current or potential applied.
However, in order to maintain the constant effective current or potential, a high-performance power supply may be required to provide high current/potential and a fast switch.
A small gap-to-sample-area ratio may cause uneven distribution of current and affect the surface topology of the plated sample.
The objects are placed in a barrel-shaped non-conductive cage and then immersed in a chemical bath containing dissolved ions of the metal that is to be plated onto them.
[8] Cleanliness is essential to successful electroplating, since molecular layers of oil can prevent adhesion of the coating.
Perfectly clean metal surfaces are hydrophilic and will retain an unbroken sheet of water that does not bead up or drain off.
[2] Micro throwing power refers to the extent to which a process can fill or coat small recesses such as through-holes.
where R is the universal gas constant, T is the operating temperature, κ is the ionic conductivity of the plating solution, F is the Faraday constant, L is the equivalent size of the plated object, α is the transfer coefficient, and i the surface-averaged total (including hydrogen evolution) current density.
[10] The Wagner number is rather difficult to measure accurately; therefore, other related parameters, that are easier to obtain experimentally with standard cells, are usually used instead.
The macro throwing power is calculated from the thickness of plating at the two cathodes when a direct current is passed for a specific period of time.
It measures useable current density range, optimization of additive concentration, recognition of impurity effects, and indication of macro throwing power capability.
As a result, the deposit is plated at a range current densities along its length, which can be measured with a Hull cell ruler.
[16] Electroplating of acid gold on underlying copper- or nickel-plated circuits reduces contact resistance as well as surface hardness.
There are a number of alternative processes to produce metallic coatings on solid substrates that do not involve electrolytic reduction: Electroplating was invented by Italian chemist Luigi Valentino Brugnatelli in 1805.
Brugnatelli used his colleague Alessandro Volta's invention of five years earlier, the voltaic pile, to facilitate the first electrodeposition.
Brugnatelli's inventions were suppressed by the French Academy of Sciences and did not become used in general industry for the following thirty years.
By 1839, scientists in Britain and Russia had independently devised metal-deposition processes similar to Brugnatelli's for the copper electroplating of printing press plates.
Research from the 1930s had theorized that electroplating might have been performed in the Parthian Empire using a device resembling a Baghdad Battery, but this has since been refuted; the items were fire-gilded using mercury.
Galvanoplastics quickly came into fashion in Russia, with such people as inventor Peter Bagration, scientist Heinrich Lenz, and science-fiction author Vladimir Odoyevsky all contributing to further development of the technology.
[18] Soon after, John Wright of Birmingham, England discovered that potassium cyanide was a suitable electrolyte for gold and silver electroplating.
Electroplating baths and equipment based on the patents of the Elkingtons were scaled up to accommodate the plating of numerous large-scale objects and for specific manufacturing and engineering applications.
The plating industry received a big boost with the advent of the development of electric generators in the late 19th century.
With the higher currents available, metal machine components, hardware, and automotive parts requiring corrosion protection and enhanced wear properties, along with better appearance, could be processed in bulk.
One of the American physicist Richard Feynman's first projects was to develop technology for electroplating metal onto plastic.

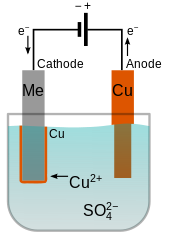
4 in sulfuric acid . A copper anode is used to replenish the electrolyte with copper cations Cu 2+
as they are plated out at the cathode.





