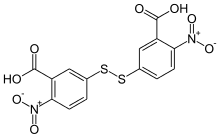
Ellman's reagent
Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB) is a colorogenic chemical used to quantify the number or concentration of thiol groups in a sample.
Thiols react with this compound, cleaving the disulfide bond to give 2-nitro-5-thiobenzoate (TNB−), which ionizes to the TNB2− dianion in water at neutral and alkaline pH.
The TNB2− is quantified in a spectrophotometer by measuring the absorbance of visible light at 412 nm, using an extinction coefficient of 14,150 M−1 cm−1 for dilute buffer solutions,[4][5] and a coefficient of 13,700 M−1 cm−1 for high salt concentrations, such as 6 M guanidinium hydrochloride or 8 M urea.
[5] Ellman's original 1959 publication estimated the molar extinction at 13,600 M−1 cm−1, and this value can be found in some modern applications of the method despite improved determinations.
[5] Ellman's reagent can be used for measuring low-molecular mass thiols such as glutathione in both pure solutions and biological samples, such as blood.