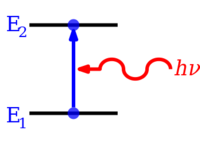
Energy level
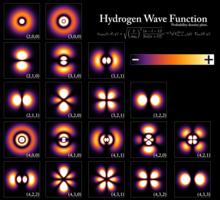
The shells correspond with the principal quantum numbers (n = 1, 2, 3, 4, ...) or are labeled alphabetically with letters used in the X-ray notation (K, L, M, N, ...).
If an atom, ion, or molecule is at the lowest possible energy level, it and its electrons are said to be in the ground state.
An energy level is regarded as degenerate if there is more than one measurable quantum mechanical state associated with it.
Informally, these states correspond to a whole number of wavelengths of the wavefunction along a closed path (a path that ends where it started), such as a circular orbit around an atom, where the number of wavelengths gives the type of atomic orbital (0 for s-orbitals, 1 for p-orbitals and so on).
Elementary examples that show mathematically how energy levels come about are the particle in a box and the quantum harmonic oscillator.
The first evidence of quantization in atoms was the observation of spectral lines in light from the sun in the early 1800s by Joseph von Fraunhofer and William Hyde Wollaston.
The energy of its state is mainly determined by the electrostatic interaction of the (negative) electron with the (positive) nucleus.
The energy levels of an electron around a nucleus are given by: (typically between 1 eV and 103 eV), where R∞ is the Rydberg constant, Z is the atomic number, n is the principal quantum number, h is the Planck constant, and c is the speed of light.
For hydrogen-like atoms (ions) only, the Rydberg levels depend only on the principal quantum number n. This equation is obtained from combining the Rydberg formula for any hydrogen-like element (shown below) with E = hν = hc / λ assuming that the principal quantum number n above = n1 in the Rydberg formula and n2 = ∞ (principal quantum number of the energy level the electron descends from, when emitting a photon).
An equivalent formula can be derived quantum mechanically from the time-independent Schrödinger equation with a kinetic energy Hamiltonian operator using a wave function as an eigenfunction to obtain the energy levels as eigenvalues, but the Rydberg constant would be replaced by other fundamental physics constants.
A simple (though not complete) way to understand this is as a shielding effect, where the outer electrons see an effective nucleus of reduced charge, since the inner electrons are bound tightly to the nucleus and partially cancel its charge.
This leads to an approximate correction where Z is substituted with an effective nuclear charge symbolized as Zeff that depends strongly on the principal quantum number.
For filling an atom with electrons in the ground state, the lowest energy levels are filled first and consistent with the Pauli exclusion principle, the Aufbau principle, and Hund's rule.
Fine structure arises from relativistic kinetic energy corrections, spin–orbit coupling (an electrodynamic interaction between the electron's spin and motion and the nucleus's electric field) and the Darwin term (contact term interaction of s shell[which?]
There is an interaction energy associated with the magnetic dipole moment, μL, arising from the electronic orbital angular momentum, L, given by with Additionally taking into account the magnetic momentum arising from the electron spin.
Due to relativistic effects (Dirac equation), there is a magnetic momentum, μS, arising from the electron spin with gS the electron-spin g-factor (about 2), resulting in a total magnetic moment, μ, The interaction energy therefore becomes Chemical bonds between atoms in a molecule form because they make the situation more stable for the involved atoms, which generally means the sum energy level for the involved atoms in the molecule is lower than if the atoms were not so bonded.
[4] In polyatomic molecules, different vibrational and rotational energy levels are also involved.
where Eelectronic is an eigenvalue of the electronic molecular Hamiltonian (the value of the potential energy surface) at the equilibrium geometry of the molecule.
Electrons can also be completely removed from a chemical species such as an atom, molecule, or ion.
Energy level transitions can also be nonradiative, meaning emission or absorption of a photon is not involved.
If an atom, ion, or molecule is at the lowest possible energy level, it and its electrons are said to be in the ground state.
[4] since c, the speed of light, equals to fλ[4] Correspondingly, many kinds of spectroscopy are based on detecting the frequency or wavelength of the emitted or absorbed photons to provide information on the material analyzed, including information on the energy levels and electronic structure of materials obtained by analyzing the spectrum.
A subsequent drop of an electron to a lower energy level can release a photon, causing a possibly coloured glow.