Enoyl CoA isomerase
Since the key step in the degradation of fatty acids with double bonds at even-numbered carbon positions also produces 3-trans-enoyl-CoA in mammals and yeasts, enoyl-CoA isomerase is technically required for their metabolism as well.
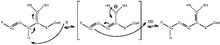
[3] The reaction mechanism is detailed in figure 1,[4] and the base that initiates the isomerization and NH groups that stabilize the intermediate are located on the active site of enoyl-coA isomerase.
[8] Also, it has been found to be a subunit of the peroxisomal trifunctional enzyme (pTFE) and contributes only to minor cleavages of the fatty acid chain.
In that sense, for many higher organisms, the mitochondrial enzyme is essential for deriving maximum energy from lipids and fueling muscles.
[13] Due to a high turnover rate, the plant peroxisomes contain a lesser amount of enoyl-CoA isomerase than their counterparts in the rat liver.
[15] This conserved region must be important for structure and function of this specific enzyme since showing equally in both E. coli and rat liver.
[4] Moreover, a glutamate residue located next to body cavities filled with water molecules and lined with hydrophobic or apolar side chains has also been identified as a part of the catalytic site.
The body cavities aid in rearranging the glutamate side chain to retain the proton and later deliver it back to the acyl-CoA, on a different carbon position.
[20] However, the human mitochondrial enoyl-CoA isomerase is a trimer and orients the fatty acid tail in a completely different direction from that seen in the hexamers.
[20] Enoyl-CoA isomerase was first identified and purified from rat liver mitochondria in the 1960s and 1970s via gel filtration and ion exchange chromatography.
[2] In the same year, the protein itself was isolated, not by affinity to rat antibody or cDNA probes,[3] but by copurification with a transferase, human glutathione S-transferases.
[22] In the attempts to examine the human enoyl-CoA isomerase in detail, the mitochondrial enzyme in the mammalian liver was identified as a potential biological marker for metabolic diseases due to its elevated levels in defective cells, and linked defects in fatty acid beta-oxidation to human diseases,[22] to be specified in the next section.
In humans, defects in the beta-oxidation mechanism result in hypoketotic hyperglycemia, a symptom of starvation, due to the inefficient utilization of fatty acids as a primary source of energy.
[9] More recent studies link hepatitis C virus (HCV) infection to defects in fatty acid degradation, specifically, to that in enoyl-CoA isomerase.