Eos (protein)
EosFP was first discovered in 2005 during a large scale screen for PAFPs (photoactivatable fluorescent proteins) within the stony coral Lobophyllia hemprichii.
[4] These variants have been successful in the tracking of cellular components without disturbing function in the host cell and maintain the same photophysical properties as wild-type Eos.
[5] Since their discovery, monomeric Eos probes (mEos) have been shown to localize in the cytosol, plasma membrane, endosomes, prevacuolar vesicles, vacuoles, the endoplasmic reticulum, golgi bodies, peroxisomes, mitochondria, invaginations, filamentous actin and cortical microtubules.
The two fluorescent forms of mEosFP (green and red) are compatible with CFP, GFP, YFP and RFP for multicolour labelling.
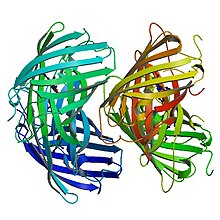
[8] According to single-molecule fluorescence spectroscopy, EosFP is tetrameric, and exhibits strong Forster resonance coupling within individual fluorophores.
[2] Like other fluorescent proteins, Eos can be used to report diverse signals in cells, tissues and organs without disturbing complex biological machinery.
Eos has 84% identical residues to Kaede, a fluorescent protein that originated in a different scleractinian coral Trachyphyllia geoffroyi, but can also be irreversibly converted from a green to red emitting form using UV light.
When this histidine residue is substituted with M, S, T or L, Eos only emits bright green light and no longer acts as a photoconvertible fluorescent protein.
[5] The observed red fluorescence occurs due to an extension of the chromophore's π-conjugation where the His-62 imidazole ring connects to the imidazolinone.
These observations suggest that the neutral form of the green chromophore, including a protonated Tyr-63 side chain, is the gateway structure for photoconversion.
When His-62 is replaced with other amino acids, EosFP loses its ability to photoconvert, providing evidence that His-62 is a necessary component of the photoconversion mechanism.
An absorption peak at 280 nm is visible due to aromatic amino acids which transfer their excitation energy to the green chromophore.
[3] These fusion constructs have been used to visualize nuclear translocation with androgen receptors, dynamics of the cytoskeleton with actin and vinculin and intranuclear protein movement with RBP.
[3] In a dual-colour labelling experiment to map the stages of mitosis, HEK293 cells were first stably transfected with tubulin-binding protein cDNA fused to EGFP for visualization of the spindle apparatus.
At the two-cell/ early gastrula stage, capped mRNA coding for a dimeric EosFP (d2EosFP) was injected into cells and locally photoconverted using fluorescence microscopy.
Developed by a team at the Janelia Farm Research Campus at Howard Hughes Medical Institute, mEos4 has higher photostability and longer imaging abilities than EosFP.
Where tdEos (tandem dimer) cannot fuse to targets such as histones, tubulin, intermediate filaments and gap junctions, and mEos (monomeric) which can only be used successfully at 30 degrees Celsius, mEos2 is an engineered variant that can fold effectively at 37 degrees Celsius and successfully label targets intolerant to fusion from other fluorescent protein dimers .
[10] Also at the Janelia Research Campus, a new fluorescent molecules known as CaMPARI (calcium-modulated photoactivatable ratiometric integrator) was developed using EosFP.
[11] The permanent green to red conversion signal was coupled with a calcium-sensitive protein, calmodulin, so that color change in the fusion construct depended on the release of calcium accompanied by neural activity.