Cytokinesis
Particular functions demand various deviations from the process of symmetrical cytokinesis; for example in oogenesis in animals the ovum takes almost all the cytoplasm and organelles.
[1] Another form of mitosis occurs in tissues such as liver and skeletal muscle; it omits cytokinesis, thereby yielding multinucleate cells (see syncytium).
The word "cytokinesis" (/ˌsaɪtoʊkaɪˈniːsɪs, -tə-, -kə-/[2][3]) uses combining forms of cyto- + kine- + -sis, Neo-Latin from Classical Latin and Ancient Greek, reflecting "cell" and kinesis ("motion, movement").
The process can be divided to the following distinct steps: anaphase spindle reorganization, division plane specification, actin-myosin ring assembly and contraction, and abscission.
[5] Faithful partitioning of the genome to emerging daughter cells is ensured through the tight temporal coordination of the above individual events by molecular signaling pathways.
A number of different species including H. sapiens, D. melanogaster and C. elegans require the central spindle in order to efficiently undergo cytokinesis, although the specific phenotype associated with its absence varies from one species to the next (for example, certain Drosophila cell types are incapable of forming a cleavage furrow without the central spindle, whereas in both C. elegans embryos and human tissue culture cells a cleavage furrow is observed to form and ingress, but then regress before cytokinesis is complete).
[5] The decline of CDK1 activity at the metaphase-anaphase transition leads to dephosphorylating of inhibitory sites on multiple central spindle components.
First of all, the removal of a CDK1 phosphorylation from a subunit of the CPC (the chromosomal passenger complex) allows its translocalization to the central spindle from the centromeres, where it is located during metaphase.
Meanwhile, the mechanism by which the spindle determines the division plane in animal cells is perhaps the most enduring mystery in cytokinesis and a matter of intense debate.
The central spindle may contribute to the specification of the division plane by promoting concentration and activation of the small GTPase RhoA at the equatorial cortex.
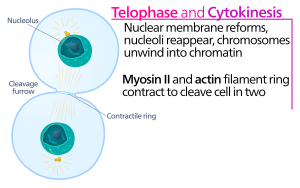
Following its assembly, contraction of the actin-myosin ring leads to ingression of the attached plasma membrane, which partitions the cytoplasm into two domains of emerging sister cells.
Myosin II uses the free energy released when ATP is hydrolyzed to move along these actin filaments, constricting the cell membrane to form a cleavage furrow.
Continued hydrolysis causes this cleavage furrow to ingress (move inwards), a striking process that is clearly visible through a light microscope.
The cytokinetic furrow ingresses until a midbody structure (composed of electron-dense, proteinaceous material) is formed, where the actin-myosin ring has reached a diameter of about 1–2 μm.
Most animal cell types remain connected by an intercellular cytokinetic bridge for up to several hours until they are split by an actin-independent process termed abscission, the last step of cytokinesis.
Abscission proceeds by removal of cytoskeletal structures from the cytokinetic bridge, constriction of the cell cortex, and plasma membrane fission.
The final step of abscission is controlled by the recruitment and polymerization of the endosomal sorting complex required for transport III (ESCRT-III), which serves to physically constrict and separate the plasma membrane of the two adjoined daughter cells.
[9] Cytokinesis must be temporally controlled to ensure that it occurs only after sister chromatids separate during the anaphase portion of normal proliferative cell divisions.
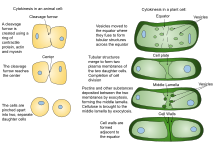
The first components to arrive are pectins, hemicelluloses, and arabinogalactan proteins carried by the secretory vesicles that fuse to form the cell plate.
Since Myosins are recruited to the medial region, the contractile forces acting on the cortex resemble a 'purse string' constriction pulling inwards.
[24] Reduction of external pressure and of surface tension (by membrane trafficking) reduce the required stabilization and constriction forces.