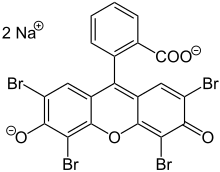
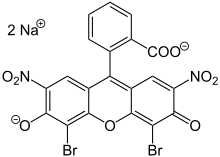
Eosin
Eosin is the name of several fluorescent acidic compounds which bind to and form salts with basic, or eosinophilic, compounds like proteins containing amino acid residues such as arginine and lysine, and stains them dark red or pink as a result of the actions of bromine on eosin.
[1][2] Eosin was named by its inventor Heinrich Caro after the nickname (Eos) of a childhood friend, Anna Peters.
For staining, eosin Y is typically used in concentrations of 1 to 5 percent weight by volume, dissolved in water or ethanol.
[11] A small concentration (0.5 percent) of acetic acid usually gives a deeper red stain to the tissue.
Eosin is also used as a red dye in inks; however, the molecule, especially that of eosin Y, tends to degrade over time, leaving behind its bromine atoms, hence causing paint incorporating such a dye to obtain a darker brown tinge over time.