Epinephrine autoinjector
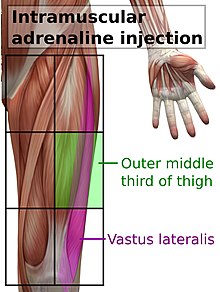
[1][2] When anaphylaxis is suspected, epinephrine solution should be given as soon as possible as an intramuscular injection, in the middle of the outer side of the thigh, which corresponds to the location of the vastus lateralis muscle.
[7] Unintentional injections are delivered to a finger or thumb around 90% of the time; they cause intense pain locally but usually completely resolve.
It was emphasized this was an emergency adaptation of the intended design for austere environments when no additional epinephrine was available.
[10] Autoinjectors were originally developed for the rapid administration of nerve gas antidotes in kits like the Mark I NAAK.
The first modern epinephrine autoinjector, the EpiPen, was invented in the mid-1970s at Survival Technology in Bethesda, Maryland, US by Sheldon Kaplan[11][12] and was first approved for marketing by the FDA in 1987.
[18] In 2001 Meridian and Dey introduced a two-pack version of the EpiPen; at that time the device had $23.9 million in annual sales and accounted for 75% of the market in the United States.
[30] In 2009, Mylan and King started marketing a new version of EpiPen with the same basic mechanism but a stronger spring, better safety features, and clearer markings and instructions; an expert for NBC News estimated that the cost to redesign the device and packaging may have been "several million dollars" and the cost to retool the manufacturing process may have "run into the double-digit millions.
[50] In 2011, Pfizer and King sued Intelliject and Sanofi after the companies filed a 505(b)(2)[51] New Drug Application for the product, then known as "e-cue";[52] Pfizer, Mylan and Sanofi settled in 2012 under a deal that allowed the device to enter the market no earlier than November 2012, pending FDA approval.
[58] In 2012, Shionogi, the manufacturer of Adrenaclick and Twinject, announced it would stop making them;[34] it had sold the rights to the NDA to a company called Amedra Pharmaceuticals.
[63] After successful lobbying from Mylan,[29] in 2013, the "School Access to Emergency Epinephrine Act" became law after passing Congress with broad and bipartisan support; it protected anyone from liability if they administered epinephrine to a child in a school (previously, only trained professionals or the affected person were allowed to administer the drug, and were open to liability), and it provided some financial incentives for schools that didn't already stock epinephrine autoinjector to start stocking them.
[72][73] The reason stated by Sanofi was that the products had been found to potentially have inaccurate dosage delivery, which may include failure to deliver drug.
[74] In February 2016, Sanofi terminated its license to manufacture and market the Auvi-Q, leaving Kaléo (Intelliject was renamed) to consider how and whether to re-introduce the device.
According to the FDA, the manufacturer of EpiPen devices failed to address known malfunctions in its auto-injectors even as hundreds of customer complaints rolled in and failures were linked to deaths.
[86][87] In an effort to address the supply shortage of EpiPens,[88] on August 21, 2018 the FDA approved extending the expiration dates on some products by four months.
[93] From 2015 to 2020 the only autoinjector marketed in Canada was EpiPen and production issues led to supply shortages during that period.
[94][95][96] During a 2018 shortage of EpiPens, Health Canada temporarily permitted the importation of Auvi-Q autoinjectors from the United States.
[93] As of September 2016[update], two EpiPens cost around $100 in France and at maximum 10€ for members of the statutory health insurance in Germany.
[43][98] As of September 2016[update], two Jext autoinjectors cost users about £8.50 (US$11.64) in Britain, and the National Health Service pays around £48 (US$65.75) in order to make them available; that price was about 17 percent less than 2013.
[29][clarification needed] In September 2016, a Silicon Valley engineering consultancy performed a teardown analysis of the EpiPen and estimated the manufacturing and packaging costs at about $10 for a two-pack.
[102] Mylan acquired the right to market the EpiPen line of epinephrine autoinjector devices from Merck KGaA as part of their 2007 deal.
[28] Heather Bresch, Mylan's CEO, saw an opportunity to increase sales in the US through marketing and advocacy, and the company launched a marketing campaign to increase awareness of the dangers of anaphylaxis for people with severe allergies that made the EpiPen brand as identified with epinephrine autoinjectors as Kleenex is for facial tissue; the company also successfully lobbied the FDA to broaden the label to include risk of anaphylaxis and in parallel, successfully lobbied Congress to generate legislation making EpiPens available in public places like defibrillators are, and hired the same people that Medtronic had worked with on defibrillator legislation to do so.
[29] Mylan's efforts to maintain its market dominance were aided when Sanofi's competing product was recalled in November 2015 and further when Teva's generic competitor was rejected by the FDA in March 2016.
[78] The last price increase sparked widespread outrage in the late summer as parents prepared to send their children back to school and went to pharmacies to get new EpiPens.
[106][107] Former Mylan CEO, Heather Bresch testified before the U.S. Senate Special Committee on Aging to argue that the price change was "fair".
[108] In response to criticism, Mylan increased financial assistance available for some patients to purchase EpiPens,[109] a gesture that was called a "classic public relations move" by Harvard Medical School professor Aaron Kesselheim.