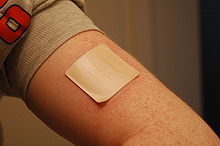
Transdermal patch
[2][3][4][5] In order to overcome restriction from the skin, researchers have developed microneedle transdermal patches (MNPs), which consist of an array of microneedles, which allows a more versatile range of compounds or molecules to be passed through the skin without having to micronize the medication beforehand.
The drug reservoir is totally encapsulated in a shallow compartment molded from a drug-impermeable metallic plastic laminate, with a rate-controlling membrane made of a polymer like vinyl acetate on one surface.
Other vapour patches on the market improve quality of sleep or aid in smoking cessation.
Embedding as many as 102-104 needles per square centimetre of patch, encapsulated or coated with intended drug, MNPs can easily pass skin tissue known as the stratum corneum which is roughly 20 μm in thickness, allowing up to the size of macromolecule to pass.
[6] MNPs were developed mainly because transdermal patch can deliver smaller size or micronized molecules such as nicotine and birth control which easily diffuse and penetrate the skin, but lack in delivering macro or large size molecules.
[6] As mentioned earlier, MNPs deliver more efficient delivery compared to topical or oral intake.
In drug delivery study, researchers want to gain faster peak concentrations (Cmax) in MNPs compared to other methods.
This value is only matched with direct injection, but with skin trauma and people with needle phobia, MNPs might be an alternative to reach roughly the same time and concentration.
Fortunately, researchers have developed a water-insoluble backing layer, making the needle last longer in the human body environment.
Meaning that inactive virus or pathogen can be introduced in the body without discomfort or skin irritation from conventional injection.
The MNPs are small and thin compared to bottles of vial, making it possible to transport in massive quantities in a single trip.
[33] Medical waste such as syringes and dirty needles are also eliminated, reducing the possibility of pathogen transmission of blood-borne disease in rural areas.
[7] In a study, measles coated MNPs might be resistant to higher temperature compared to vial transport.
Higher temperature resistance is a safe bet in low income countries, where there is no such luxury for refrigeration.
However, the study in MNPs measles vaccine is still under development, but opening possibilities in the future for other types of vaccines[33] Skin treatment including face whitening agent and dark eye circles serum can also incorporated in MNPs.
By measuring the melanin (dark or black pigment found on the skin) index, subjects that are treated with whitening agents coated in MNPs show lower melanin index, compared to the whitening essence (topical) group.
[1] Because most of MNPs applications are still under development, it is important to note the long effect of the efficiency of the drug deliveries.
Prior to sale in the United States, any transdermal patch product must apply for and receive approval from the Food and Drug Administration, demonstrating safety and efficacy for its intended use.