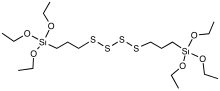
Cross-link
When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies.
Intermediate cross-link densities transform gummy polymers into materials that have elastomeric properties and potentially high strengths.
A class of polymers known as thermoplastic elastomers rely on physical cross-links in their microstructure to achieve stability, and are widely used in non-tire applications, such as snowmobile tracks, and catheters for medical use.
Alkyd enamels, the dominant type of commercial oil-based paint, cure by oxidative crosslinking after exposure to air.
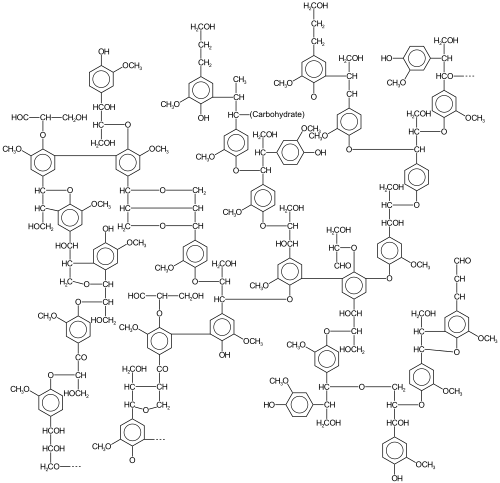
Lignin is a highly crosslinked polymer that comprises the main structural material of higher plants.
[12] In proteins, crosslinks are important in generating mechanically stable structures such as hair and wool, skin, and cartilage.
The process of applying a permanent wave to hair involves the breaking and reformation of disulfide bonds.
The neutralizer is typically an acidic solution of hydrogen peroxide, which causes new disulfide bonds to form, thus permanently fixing the hair into its new configuration.
The zero-length carbodiimide crosslinker EDC functions by converting carboxyls into amine-reactive isourea intermediates that bind to lysine residues or other available primary amines.
SMCC or its water-soluble analog, Sulfo-SMCC, is commonly used to prepare antibody-hapten conjugates for antibody development.
[19] Typical reagents are ammonium persulfate (APS), an electron acceptor, the photosensitizer tris-bipyridylruthenium (II) cation ([Ru(bpy)3]2+).
Upon exposure to ultraviolet light, the diazirines are activated and bind to interacting proteins that are within a few ångströms of the photo-reactive amino acid analog (UV cross-linking).