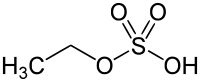
Ethyl sulfate
This substance was studied contemporaneously with ether by German alchemist August Siegmund Frobenius in 1730,[1] subsequently by French chemists Fourcroy in 1797 and Gay-Lussac in 1815.
[4] In 1827, French chemist and pharmacist Félix-Polydore Boullay (1806-1835) along with Jean-Baptiste André Dumas noted the role of ethyl sulfate in the preparation of diethyl ether from sulfuric acid and ethanol.
[11] Ethyl sulfate can be produced in a laboratory setting by reacting ethanol with sulfuric acid under a gentle boil, while keeping the reaction below 140 °C.
If the temperature exceeds 140 °C, the ethyl sulfate product tends to react with residual ethanol starting material, producing diethyl ether.
[13] Original: Jag skall derföre, för att begagna en i kemien välkänd härledning, kalla den kroppars katalytiska kraft, sönderdelning genom denna kraft katalys, likasom vi med ordet analys beteckna åtskiljandet af kroppars beståndsdelar medelst den vanliga kemiska frändskapen.Translation: I shall, therefore, to employ a well-known derivation in chemistry, call [the catalytic] bodies [i.e., substances] the catalytic force and the decomposition of [other] bodies by this force catalysis, just as we signify by the word analysis the separation of the constituents of bodies by the usual chemical affinities.