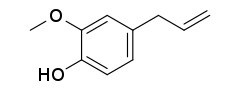
Eugenol
Eugenol /ˈjuːdʒɪnɒl/ is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds.
[2] It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf.
Like many other anesthetic agents, these 2-alkyl(oxy)phenols act as positive allosteric modulators of the GABAA receptor.
Although eugenol and thymol are too toxic and not potent enough to be used clinically, these findings led to the development of 2-substituted phenol anesthetic drugs, including propanidid (later withdrawn) and the widely used propofol.
[12] Eugenol and the structurally similar myristicin, have the common property of inhibiting MAO-A and MAO-B in vitro.
[28][29] Eugenol is an ingredient in some fungicides and weed control products used in agricultural practices in the European Union.
[2] Lesser side effects of eugenol toxicity that may not be considered a full overdose: Sedation, dizziness, hallucinations, delirium, mild respiratory depression, nausea, and muscle spasms.
[17] An overdose is possible, causing a wide range of symptoms, such as hematuria (blood in urine), convulsions, diarrhea, unconsciousness, heavy respiratory depression, tachycardia (rapid heart rate), or acute kidney injury.