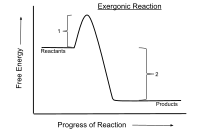
Exergonic reaction
For processes that take place in a closed system at constant pressure and temperature, the Gibbs free energy is used, whereas the Helmholtz energy is relevant for processes that take place at constant volume and temperature.
Any reaction occurring at constant temperature without input of electrical or photon energy is exergonic, according to the second law of thermodynamics.
For instance, the disproportionation of hydrogen peroxide releases free energy but is very slow in the absence of a suitable catalyst.
[2] More generally, the terms exergonic and endergonic relate to the free energy change in any process, not just chemical reactions.
By contrast, the terms exothermic and endothermic relate to an enthalpy change in a closed system during a process, usually associated with the exchange of heat.