Exopolyphosphatase
Exopolyphosphatase (PPX) is a phosphatase enzyme which catalyzes the hydrolysis of inorganic polyphosphate, a linear molecule composed of up to 1000 or more monomers linked by phospho-anhydride bonds.
[3] It is especially important for maintenance of appropriate levels of intracellular polyphosphate, which has been implicated in a variety of cellular functions including response to stressors such as deficiencies in amino acids, orthophosphate, or nitrogen, changes in pH, nutrient downshift, and high salt, and as an inorganic molecular chaperone.
[1] PPX is mixed with a known quantity of labeled polyphosphate, and the hydrolysis reaction is stopped with perchloric acid (HClO4).
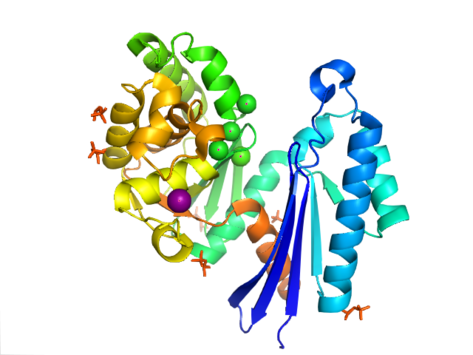
In Aquifex aeolicus it contains a ribonuclease H-like motif that is made up of a five-stranded β-sheet with the second strand antiparallel to the rest.
This configuration shares similar features to other members of this superfamily, including the N-terminal and C-terminal domains being separated by two alpha-helices centered on the structure.
[9] To date, 4 structures have been solved for this class of enzymes, with Protein Data Bank accession codes 1T6C, 1T6D, 1U6Z, and 2FLO.
[9] Exopolyphosphatase cleaves a terminal phosphate off of polyphosphate through the amino acid side chains of glutamate and lysine.
The oxygen that was previously bridging the two phosphate atoms then abstracts a hydrogen from the nearby lysine residue.
It has also been shown to be involved with cell membrane formation and function, enzyme regulation, and gene transcriptional control.
In mammals, polyphosphates are involved with blood coagulation and inflammation, immune response, bone tissue development, and brain function.
[15] Conversely, yeast strains that have higher levels exopolyphosphatase enzyme are shown to have no obvious growth defects under phosphate deficiency or excess phosphate conditions, however the level of polyphosphate in the yeast was much lower due to the increased number of enzymes breaking the polyphosphate chains down.