Facioscapulohumeral muscular dystrophy
Per the name, FSHD tends to sequentially weaken the muscles of the face, those that position the scapula, and those overlying the humerus bone of the upper arm.
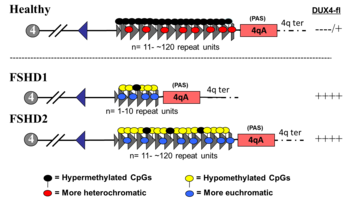
[4] Mutations of FSHD cause inadequate DUX4 repression by unpacking the DNA around DUX4, making it accessible to be copied into messenger RNA (mRNA).
[42][43] These abnormalities of arterioles usually do not affect vision or health, although a severe form of it mimics Coat's disease, a condition found in about 1% of FSHD cases and more frequently associated with large 4q35 deletions.
[2][8][50] The mechanism of failed DUX4 repression is hypomethylation of DUX4 and its surrounding DNA on the tip of chromosome 4 (4q35), allowing transcription of DUX4 into messenger RNA (mRNA).
[27] Disease can only result when a mutation is present in combination with select, commonly found variations of 4q35, termed haplotype polymorphisms, which are roughly dividable into the groups 4qA and 4qB.
[51] A 4qA haplotype polymorphism, often referred to as a 4qA allele, is necessary for disease, as it contains a polyadenylation sequence that allows DUX4 mRNA to resist degradation long enough to be translated into DUX4 protein.
[52] Each D4Z4 repeat is 3.3 kilobase pairs (kb) long and is the site of epigenetic regulation, containing both heterochromatin and euchromatin structures.
[14] It has been proposed that FSHD1 undergoes anticipation, a phenomenon primarily associated with trinucleotide repeat disorders in which disease manifestation worsens with each subsequent generation.
A deactivating mutation of one of several DNA methylation genes is required for FSHD2, which contributes to hypomethylation of a borderline shortened D4Z4 repeat array, at which the genetic mechanism converges with FSHD1.
[75] Estrogen seems to play a role in modifying DUX4 protein effects on muscle differentiation, which could explain why females are less affected than males, although it remains unproven.
[36] Medical imaging (CT and MRI) have shown muscle involvement not readily apparent otherwise[37] Tortuosity of the retinal arterioles, and less often microaneurysms and telangiectasia, are commonly found in FSHD.
[42] It has been hypothesized that retinopathy is due to DUX4-protein-induced modulation of the CXCR4–SDF1 axis, which has a role in endothelial tip cell morphology and vascular branching.
[90] These methods, which physically measure the size of the D4Z4 repeat array, require specially prepared high-quality and high molecular weight genomic DNA (gDNA) from serum, increasing cost and reducing accessibility to testing.
[91] Restriction fragment length polymorphism (RFLP) analysis was the first genetic test developed and is still used as of 2020, although it is being phased out by newer methods.
[92] The proximal portion has a sequence of DNA stainable by the probe p13E-11, which is commonly used to visualize the EcoRI fragment during southern blot.
Cognitive behavioral therapy (CBT) has been shown to reduce chronic fatigue in FSHD, and it also decelerates fatty infiltration of muscle when directed towards increasing daily activity.
Scapular fixation is the restriction and stabilization of the position of the scapula, putting it in closer apposition to the rib cage and reducing winging.
[2] The AAN states that scapular fixation can be offered cautiously to select patients after balancing these benefits against the adverse consequences of surgery and prolonged immobilization.
[108] Another form of scapular fixation, although not commonly done in FSHD, is tendon transfer, which involves surgically rearranging the attachments of muscles to bone.
[115] Ability to smile can theoretically be restored with a tendon transfer, with donors such as a portion of the temporalis muscle, although evidence is lacking in FSHD.
[121] A single review found that weakness worsens, without recovery, in 12% of mothers with FSHD during pregnancy, although this might be due to weight gain or deconditioning.
[18][17] In their paper of 1886, Landouzy and Dejerine drew attention to the familial nature of the disorder and mentioned that four generations were affected in the kindred that they had investigated.
With increasing confidence in this work, researchers proposed the first a consensus view in 2014 of the pathophysiology of the disease and potential approaches to therapeutic intervention based on that model.
[133] DNA sequencing within D4Z4 units shows they contain an open reading frame corresponding to two homeobox domains, but investigators conclude that D4Z4 is unlikely to code for a functional transcript.
Stable DUX4 mRNA is transcribed only from the most distal D4Z4 unit, which uses an intron and a polyadenylation signal provided by the flanking pLAM region.
"One of the report's co-authors, Silvère van der Maarel of the University of Leiden, stated that[citation needed] "It is amazing to realize that a long and frustrating journey of almost two decades now culminates in the identification of a single small DNA variant that differs between patients and people without the disease.
[153] A subsequent study using a larger number of samples identified DUX4-fl expression in myogenic cells and muscle tissue from unaffected relatives of FSHD patients, per se, is not sufficient to cause pathology, and that additional modifiers are determinants of disease progression.
[154] Transgenic mice carrying D4Z4 arrays from an FSHD1 allele (with 2.5 D4Z4 units), although lacking an obvious FSHD-like skeletal muscle phenotype, are found to recapitulate important genetic expression patterns and epigenetic features of FSHD.
They describe the consensus mechanism of pathophysiology for FSHD as an "inefficient repeat-mediated epigenetic repression of the D4Z4 macrosatellite repeat array on chromosome 4, resulting in the variegated expression of the DUX4 retrogene, encoding a double-homeobox transcription factor, in skeletal muscle.
[19] Compounds were trialed with goals of increasing muscle mass, decreasing inflammation, or addressing provisional theories of disease mechanism.




| CEN | centromeric end | TEL | telomeric end |
| NDE box | non-deleted element | PAS | polyadenylation site |
| triangle | D4Z4 repeat | trapezoid | partial D4Z4 repeat |
| white box | pLAM | gray boxes | DUX4 exons 1, 2, 3 |
| arrows | |||
| corner | promoters | straight | RNA transcripts |
| black | sense | red | antisense |
| blue | DBE-T | dashes | dicing sites |