Ferredoxin—NADP(+) reductase
[4] The glutamate residue is highly conserved because it both stabilizes the semiquinone form of FAD and is a proton donor/acceptor in the reaction.
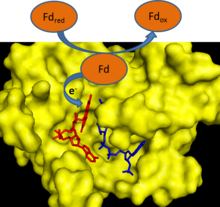
[6] This reaction can also operate in reverse to generate reduced ferredoxin, which can then be used in a variety of biosynthetic pathways.
[2] Electron cycling from ferredoxin to NADPH only occurs in the light in part because FNR activity is inhibited in the dark.
[11] In nonphotosynthetic organisms, the FNR primarily works in reverse to provide reduced ferredoxin for various metabolic pathways.
These pathways include nitrogen fixation, terpenoid biosynthesis, steroid metabolism, oxidative stress response, and iron–sulfur protein biogenesis.
[7] FNR is a soluble protein that is found both free in the chloroplast stroma and bound to the thylakoid membrane.
[16] The plastidic FNRs in plants have also evolved to have a high degree of substrate specificity for NADP+ over NAD+; studies of amino acid mutations have shown that the terminal tyrosine residue in plastidic FNRs plays a key role in this substrate specificity.
[16] Several major human diseases are caused by the obligate intracellular protozoan parasites in the phylum Apicomplexa.
The apicoplast organelle in these organisms is believed to have come from an endosymbiotic event in which an ancestral protozoan engulfed an algal cell.
[7] These apicoplasts contain plant-like FNRs that the protozoan uses to generate reduced ferredoxin, which is then used as a reductant in essential biosynthetic pathways.