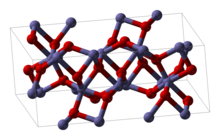
Iron(III) oxide
It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry.
It is ferromagnetic and finds application in recording tapes,[12] although ultrafine particles smaller than 10 nanometers are superparamagnetic.
[13] The epsilon (ε) phase is rhombic, and shows properties intermediate between alpha and gamma, and may have useful magnetic properties applicable for purposes such as high density recording media for big data storage.
[citation needed] Research has revealed epsilon iron(III) oxide in ancient Chinese Jian ceramic glazes, which may provide insight into ways to produce that form in the lab.
[15][non-primary source needed] Additionally, at high pressure an amorphous form is claimed.
[5][non-primary source needed] Molten Fe2O3 is expected to have a coordination number of close to 5 oxygen atoms about each iron atom, based on measurements of slightly oxygen deficient supercooled liquid iron oxide droplets, where supercooling circumvents the need for the high oxygen pressures required above the melting point to maintain stoichiometry.
When alkali is added to solutions of soluble Fe(III) salts, a red-brown gelatinous precipitate forms.
Partial reduction with hydrogen at about 400 °C produces magnetite, a black magnetic material that contains both Fe(III) and Fe(II):[18] Iron(III) oxide is insoluble in water but dissolves readily in strong acid, e.g., hydrochloric and sulfuric acids.
Rouge cuts more slowly than some modern polishes, such as cerium(IV) oxide, but is still used in optics fabrication and by jewelers for the superior finish it can produce.
Rouge is sold as a powder, paste, laced on polishing cloths, or solid bar (with a wax or grease binder).
Its use in computer disks was superseded by cobalt alloy, enabling thinner magnetic films with higher storage density.
[25] However, its efficacy is limited by a short diffusion length (2–4 nm) of photo-excited charge carriers[26] and subsequent fast recombination, requiring a large overpotential to drive the reaction.
[27] Research has been focused on improving the water oxidation performance of Fe2O3 using nanostructuring,[25] surface functionalization,[28] or by employing alternate crystal phases such as β-Fe2O3.
[29] Calamine lotion, used to treat mild itchiness, is chiefly composed of a combination of zinc oxide, acting as astringent, and about 0.5% iron(III) oxide, the product's active ingredient, acting as antipruritic.