Iron-binding proteins
[4] A well-known family of iron-dependent enzymes include oxygenases that facilitate hydroxyl group addition of one or both atoms from o2.
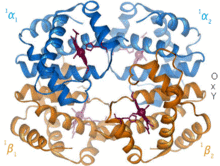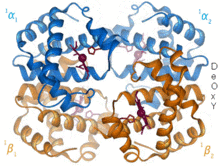
An example of this is in hemoglobin, where the porphyrin works together with a histidine side chain and a bound O2 molecule, forming an octahedral complex.
Each of the four monomeric units contain a heme prosthetic group in which a ferric cation is bound between four nitrogen atoms of a porphyrin ring.
Cytochromes are heme-containing enzymes that act as single-electron transporters, most notably as electron shuttles in oxidative phosphorylation and photosynthesis.
[10] These proteins act as electron shuttles by switching the oxidation state of the heme iron atom between ferrous (Fe2+) and ferric (Fe3+).
Various cytochromes in combination with other redox-active molecules form a gradient of standard reduction potentials that increases the efficiency of energy coupling during electron-transfer events.
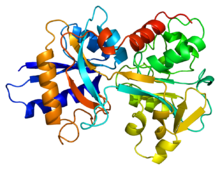
Lactoferrin is a member of the transferrin family and is the predominant protein found in mammal exocrine secretions, such as tears, milk, and saliva.
Each pocket contributes four amino acids (two tyrosines, one histidine, and one aspartate) and, along with two carbonate or bicarbonate anions, forms a six-membered coordinate around the iron cation.
It is this specific combination that makes lactoferrin's iron affinity 300 times greater than transferrin.
It is found in the highest concentration of 150 ng/mL in human colostrum (the type of milk produced at the end stages of pregnancy), providing much needed immune support to newly born infants.
It is now known, however, that the major antimicrobial driving force lies in the bactericidal properties of its iron-bound pocket and a specific peptide lactoferricin located at the N-lobe.
[17] Additionally, upon cleavage of lactoferrin by trypsin, the peptide lactoferricin is produced which binds to H+-ATPase, disrupting proton translocation and ultimately killing the cell.
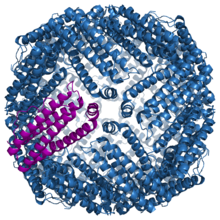
Ferritin is a large protein composed of 24 subunits surrounding a core full of iron atoms.