Ferruginol
Specifically, it is a diterpene of the abietane chemical class, meaning it is characterized by three fused six-membered rings and alkyl functional groups.
Ferruginol was first identified in 1939 by Brandt and Neubauer as the main component in the resin of the Miro tree (Podocarpus ferrugneus)[1] and has since been isolated from other conifer species in the families Cupressaceae and Podocarpaceae.
Microbial and abiotic degradation make it so most conifer biomarkers cannot be linked to specific species, so it is especially useful to find resinous samples that are able to provide more detailed identification.
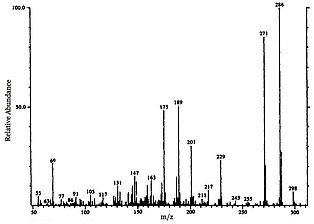
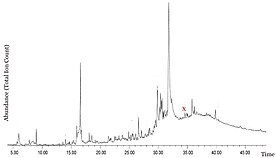
[2] The aromatic fractions are then analyzed using gas chromatography–mass spectrometry (GC-MS), and library data along with fragmentation patterns are used to identify the molecular makeup of each notable peak and their relative concentration in the sample.
[6] Additionally, time-of-flight secondary ion mass spectrometry (TOF-SIMS) has been used in combination with GC-MS with samples collected from still living organisms for surface imaging and depth profiling.
[12] Beyond anti-cancer activity, studies with mice showed that ferruginol had anti-inflammatory properties against induced ulcerative colitis[13] and acted as a gastroprotective agent against gastro lesions.
To better understand the paleoflora, researchers at Universidade Federal Rural de Pernambuco analyzed amber resin from the black shales that make up the collection site.
Palynological content had been used to date the Ipubi Formation as Aptian-Albian (125–100.5 mya), and the amber samples were thought to be allochthonous, having swept in from nearby conifer sources.
Diterpenoids of the abietanic class were the most abundant in the amber, though they are widely present in all conifer families and therefore less useful in identifying specific contributing species.