Firefly luciferin
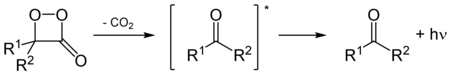
[3] As with other luciferins, oxygen is essential for the luminescence mechanism, which involves the decomposition of a cyclic peroxide to produce excited-state molecules capable of emitting light as they relax to the ground state.
The luciferin was first isolated and purified in 1949 from a large amount of specimens, though it would be several years until a procedure was developed to crystallize the compound in high yield.
This, along with the synthesis and structure elucidation, was accomplished by Dr. Emil H. White at the Johns Hopkins University, Department of Chemistry.
The luciferin could be effectively extracted using ethyl acetate at low pH from powder of approximately 15,000 firefly lanterns.
[9] Alkaline solutions caused a redshift of the absorption likely due to deprotonation of the hydroxyl group on the benzothiazole, but did not affect the fluorescence emission.