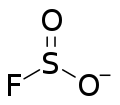
Fluorosulfite
[4] β-CsSO2F converts to α-CsSO2F when heated to 110 °C for a couple of days but remains stable below 50 °C.
[4] In solid ionic fluorosulfites, the ion is not fixed in orientation and continuously turns around resulting in dynamic disorder.
When cooled the rate of rotation slows, and can be frozen in place, resulting in static disorder.
[4] Fluorosulfite is isoelectronic with chloryl fluoride (ClO2F) and in compounds it resembles chlorate (ClO3−).
[4] The heat of formation from fluoride (F−) and sulfur dioxide (SO2) is 50 kcal mol−1.