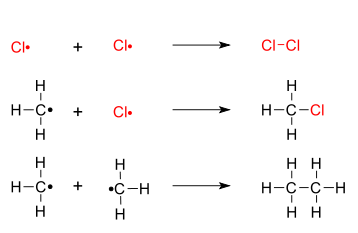
Free-radical halogenation
This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of UV light.
The relative rates at which different halogens react vary considerably:[citation needed] Radical fluorination with the pure element is difficult to control and highly exothermic; care must be taken to prevent an explosion or a runaway reaction.
With chlorine the reaction is moderate to fast; with bromine, slow and requires intense UV irradiation; and with iodine, it is practically nonexistent and thermodynamically unfavored.
[dubious – discuss][citation needed] Bond dissociation energies strongly influence any radical process and in a few unusual cases, free-radical halogenation can regioselect.
Indeed, allylic and benzylic hydrogens have bonds much weaker than alkanes, and are selectively replaced in the Wohl-Ziegler reaction.
Generally, N-haloamines in sulfuric acid (but not other haloradical sources) halogenate alkane chains at penultimate carbons (e.g. pentane to 2-halopentane), chains terminating in only carboxylic acids at the center, and bridged compounds at the bridgehead.
The rates are generally constant across reactions and predict product distributions with relatively high accuracy.