Electron microscope
[2] One significant step was the work of Hertz in 1883[3] who made a cathode-ray tube with electrostatic and magnetic deflection, demonstrating manipulation of the direction of an electron beam.
In 1931, Max Knoll and Ernst Ruska[12][13] successfully generated magnified images of mesh grids placed over an anode aperture.
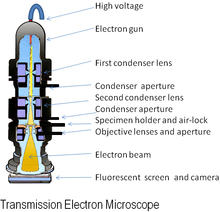
The device, a replicate of which is shown in the figure, used two magnetic lenses to achieve higher magnifications, the first electron microscope.
He stated in a very brief article in 1932[16] that Siemens had been working on this for some years before the patents were filed in 1932, claiming that his effort was parallel to the university development.
[23] Although current transmission electron microscopes are capable of two million times magnification, as scientific instruments they remain similar but with improved optics.
[26] By the early 1980s improvements in mechanical stability as well as the use of higher accelerating voltages enabled imaging of materials at the atomic scale.
[27][28] In the 1980s, the field emission gun became common for electron microscopes, improving the image quality due to the additional coherence and lower chromatic aberrations.
The 2000s were marked by advancements in aberration-corrected electron microscopy, allowing for significant improvements in resolution and clarity of images.
In X-ray crystallography, crystals are commonly visible by the naked eye and are generally in the hundreds of micrometers in length.
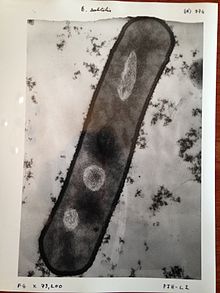
Materials to be viewed in a transmission electron microscope (TEM) may require processing to produce a suitable sample.
The choice of workflow will be highly dependent on the application and the requirements of the corresponding scientific questions, such as resolution, volume, nature of the target molecule, etc.
For example, images from light and electron microscopy of the same region of a sample can be overlaid to correlate the data from the two modalities.
Another example is high resolution mass spectrometry (ion microscopy), which has been used to provide correlative information about subcellular antibiotic localisation,[59] data that would be difficult to obtain by other means.
[60] An early example of these ‘volume EM’ workflows was simply to stack TEM images of serial sections cut through a sample.
The next development was virtual reconstruction of a thick section (200-500 nm) volume by backprojection of a set of images taken at different tilt angles - TEM tomography.
More recently, back scattered electron (BSE) images can be acquired of a larger series of sections collected on silicon wafers, known as SEM array tomography.
The increased volume available in these methods has expanded the capability of electron microscopy to address new questions,[60] such as mapping neural connectivity in the brain,[65] and membrane contact sites between organelles.
Microscopes designed to achieve high resolutions must be housed in stable buildings (sometimes underground) with special services such as magnetic field canceling systems.
Samples of hydrated materials, including almost all biological specimens, have to be prepared in various ways to stabilize them, reduce their thickness (ultrathin sectioning) and increase their electron optical contrast (staining).