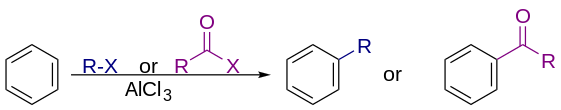Friedel–Crafts reaction
[2][3][4][5] In commercial applications, the alkylating agents are generally alkenes, some of the largest scale reactions practiced in industry.
The reaction typically employs a strong Lewis acid, such as aluminium chloride as catalyst, to increase the electrophilicity of the alkylating agent.
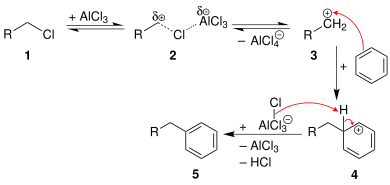
In the case of primary alkyl halides, the carbocation-like complex (R(+)---X---Al(-)Cl3) will undergo a carbocation rearrangement reaction to give almost exclusively the rearranged product derived from a secondary or tertiary carbocation.
Because, however, the product ketone forms a rather stable complex with Lewis acids such as AlCl3, a stoichiometric amount or more of the "catalyst" must generally be employed, unlike the case of the Friedel–Crafts alkylation, in which the catalyst is constantly regenerated.
Due to the electron-withdrawing effect of the carbonyl group, the ketone product is always less reactive than the original molecule, so multiple acylations do not occur.
Also, there are no carbocation rearrangements, as the acylium ion is stabilized by a resonance structure in which the positive charge is on the oxygen.
Thus, synthesis of benzaldehyde through the Friedel–Crafts pathway requires that formyl chloride be synthesized in situ.
Simple ketones that could be obtained by Friedel–Crafts acylation are produced by alternative methods, e.g., oxidation, in industry.
For example, the classical synthesis of deoxybenzoin calls for 1.1 equivalents of AlCl3 with respect to the limiting reagent, phenylacetyl chloride.
[15] Arenes react with certain aldehydes and ketones to form the hydroxyalkylated products, for example in the reaction of the mesityl derivative of glyoxal with benzene:[16] As usual, the aldehyde group is more reactive electrophile than the phenone.
[26] Examples are the synthesis of thymolphthalein (a pH indicator) from two equivalents of thymol and phthalic anhydride: A reaction of phthalic anhydride with resorcinol in the presence of zinc chloride gives the fluorophore fluorescein.